The list of predators which eat fairy shrimp is long. They are discussed here in the following order.
Fish Predators
Salamander and Frog Predators
Amphipod Predators (Crustacea: class Malacostraca, superorder Peracarida)
Copepod Predators (Crustacea: class Maxillopoda, subclass Copepoda)
Ostracod Predators (Crustacea: class Ostracoda)
Tadpole Shrimp Predators (Crustacea: class Branchiopoda, order Notostraca)
Insect Predators Overview
___ Dytiscid Predators (Hexapoda: class Insecta, order Coleoptera, family Dytiscidae)
___ Backswimmer Predators (Hexapoda: class Insecta, order Hemiptera, sub-order Heteroptera, family Notonectidae)
___ Water Boatmen Predators (Hexapoda: class Insecta, order Hemiptera, sub-order Heteroptera, family Corixidae)
___ Dragonfly and Damselfly Predators (Hexapoda: class Insecta, order Odonata)
___ Caddisfly Predators (Hexapoda: class Insecta, order Trichoptera)
___ Chaoborus Predators (Hexapoda: class Insecta, order Diptera, family Chaoboridae)
___ Other Insect Predators
Turbellarian Flatworm Predators (phylum Platyhelminthes: class Turbellaria)
Bird Predators
Fairy Shrimp Predators (Branchiopoda: Anostraca)
Human Predators
Fish Predators
Fish are at the top of the predator list for fairy shrimp. Fairy shrimp are easily gobbled up by fast-swimming fish. Unfortunately for fairy shrimp, the natural distribution of fish has been greatly expanded by humans. Humans have stocked and continue to stock fish in just about every body of water that could possibly support fish and some that can’t.
Rare instances of fairy shrimp co-occurring with fish have been reported. In one case, fingerling northern pike were seen to be eating Chirocephalus bundyi in a pond of 3.2 hectares (8 acres) in the sand hills of Nebraska (McCarraher, 1970). Adult pike had recently reached the pond through a connecting stream from a bigger lake without fairy shrimp and had deposited eggs which subsequently hatched. Ironically, C. bundyi lived in the ephemeral fish rearing ponds at Valentine State Fish Hatchery at the time. They hatched, matured, and died before the fingerlings could eat them (McCarraher, 1970). Branchinecta lindahli were found with fathead minnows in a pond of 2 hectares (5 acres), also in the sand hills, on April 20 (McCarraher, 1970). None were found on May 25. As the land owner had stocked the pond to provide bait for fishing, the minnows were not recently introduced. The fairy shrimp may have been the result of a new colonization attempt. Whether they persisted or not was not reported.
4 small lakes in the French Pyrenees that freeze to the bottom in winter have populations of Chirocephalus diaphanus (Beladjal and others, 2007a). In the summer, newly hatched trout are small enough to swim up streams that connect the fairy shrimp lakes to others with fish. By the time the fish get to the lakes, the fairy shrimp have matured, thus assuring the next generation. Any that are still alive are eaten by the fish. Fish in the fairy shrimp lakes do not survive the winter so the fairy shrimp populations persist in spite of regular fish predation (Beladjal and others, 2007a).
Predators of Fairy Shrimp – top
Salamander and Frog Predators
Continuing with vertebrate predators, Moore (1969, p. R178) listed salamanders and tadpoles. Pennak (1978, p. 337) listed “Amphibia” as “enemies” of large branchiopods. Fairy shrimp are “readily eaten by amphibians” (Kaestner, 1970, p. 90).
A web page by the National Park Service says that the western tiger salamander, which is in Wyoming and Colorado, eats “adult insects, insect nymphs and larvae, small aquatic invertebrates, frogs and other small vertebrates” (www.nps.gov/yell/learn/nature/western-tiger-salamander.htm). Fairy shrimp could easily fit into that list.
Dexter (1959, p. 561) maintained that fairy shrimp “can usually withstand predation from amphibians and carnivorous insects”. However, of 24 ponds sampled by Dodson (1970) at the Mexican Cut Nature Reserve in Colorado, 15 contained salamanders and no fairy shrimp and 8 contained fairy shrimp and no salamanders. No ponds had both. Later, Bohonak and Whiteman (1999) confirmed the exclusive relation of salamanders and fairy shrimp from permanent ponds at Mexican Cut with fairy shrimp found in only 1 of 8 permanent ponds with salamanders. The same relation did not hold for temporary ponds because the fairy shrimp could hatch and mature before enough salamanders wandered by from nearby permanent ponds to eat them all. Salamanders were seen at 7 of the 8 temporary ponds with fairy shrimp (Bohonak and Whiteman, 1999).
I haven’t seen salamanders in Nevada. In Wyoming, I saw salamanders in ephemeral ponds with or without fairy shrimp. They were in the following ponds that have fairy shrimp.
- Bull Canyon Pond (Antelope Hills)
- Blackbird Pond (Antelope Hills)
- Salamander Pond (Antelope Hills)
- Beaver Rim Quack Pond (Granite Mountains)
- 431 Steele Roadside Pond (Bighorn Basin)
I saw salamanders in the following ponds that don’t have fairy shrimp.
- Axolotl Pond (Antelope Hills)
- Upper Oregon Gulch Pond (Antelope Hills)
- “Stinking Springs Reservoir” (Antelope Hills)
- Pipeline By The Road Pond (Great Divide Basin)
- Killpecker Dunes Ponds (Killpecker Dunes)
- Upper Joe Henry Fork Pond (Bighorn Basin)
- “Cottonwood Trail Reservoir” (Bighorn Basin)
- Buttress Pond (Wind River Mountains)

A big salamander at the bottom of Bull Canyon Pond (Antelope Hills). Bull Canyon Pond had fairy shrimp at the time.
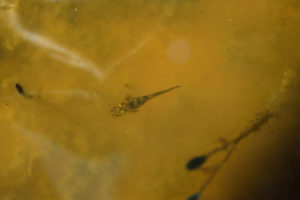
A salamander larva with gills swimming in yellowish water of Bull Canyon Pond. Bull Canyon Pond had fairy shrimp at the time.
Frogs are opportunistic predators. What they eat depends on what is available. I could find no adequately referenced or comprehensive web pages on frog diets but most indicate that tadpoles primarily eat floating algae and adults primarily eat insects. Beetles are sometimes mentioned but “insects” seems to be mostly flying insects. Adult frogs also eat aquatic insect larvae and, if big enough, smaller frogs and fish. Tadpoles less commonly eat other tadpoles and aquatic animals smaller than themselves. This is sufficient to establish that frogs are a threat to fairy shrimp.
I saw frogs or tadpoles in the following ponds that have fairy shrimp.
- “Coyote Lake” (Antelope Hills)
- Avocet Lake Southwest Pond (Antelope Hills)
- Small Rare Plant Habitat Pond (Pine Grove Hills)
- “Burnt Lake” Mud Bar Pond (Middle Washoe County)
- “Steer Lake” (Middle Washoe County)
- “Dry Steer Lake” (Middle Washoe County)
- Beauty Peak West Pond (Bodie Hills)
I saw frogs or tadpoles in the following ponds without fairy shrimp.
- Killpecker Dunes Ponds (Killpecker Dunes)
- Upper Joe Henry Fork Pond (Bighorn Basin)
- “Cottonwood Trail Reservoir” (Bighorn Basin)
- Wheeler Peak Pond (Sweetwater Mountains)
- Virginia Creek Pale Green Pond (East-Central Sierra Nevada)
- Ruby Pony Express Station Pond (Ruby Valley)
- Pegleg Breached Dam Stock Pond (Hays Canyon Range)
- Pegleg Butterfly Stock Pond (Hays Canyon Range)

A northern leopard frog (Rana pipiens) in “Coyote Lake”, Antelope Hills, which has fairy shrimp.

3 frogs (probably Pacific tree frog, Pseudacris regilla) that still have tails are now motionless on the pond bottom hoping I can’t see them. The head of the paler one in the sunlight is masked by a grass stem above the water. Other frogs at this pond (Virginia Pale Green Pond, East-Central Sierra Nevada) have lost their tails and are hopping around in the grass. This pond doesn’t have fairy shrimp.
Fairy shrimp may be able to co-exist with frogs in some ponds by hatching weeks earlier than tadpoles. I saw abundant fairy shrimp less than 10 mm long in Small Rare Plant Habitat Pond (Pine Grove Hills ) and no tadpoles on April 24, 2019, when snow was still on the ground. On July 13, 2023, when numerous flowers were blooming, I saw only one 25-mm long fairy shrimp during a 30-minute wading search. There were abundant tadpoles, many of which had short legs.
Predators of Fairy Shrimp – top
Amphipod Predators
Amphipods are fast-swimming, shrimp-like crustaceans with hard exoskeletons, 2 pairs of antennae, and 10 leg-like pereopods and pleopods. They are commonly known as scuds or sideswimmers (Thorp and Covich, 2001, p. 788-789). They belong to the superorder Peracarida and while juvenile peracaridians eat algae and bacteria, adults are more opportunistic (Thorp and Covich, 2001, p. 792). In ponds without fish, amphipods consume “phytoplankton and zooplankton”. “[I]ntraguild predation and cannibalism are also thought to be relatively common” in some species of the genus Gammarus (Thorp and Covich, 2001, p. 792). These characteristics suggest amphipods could be significant predators of fairy shrimp.
Moore (1963) identified amphipods as predators of fairy shrimp in a Louisiana pond.
In Wyoming ponds, I more commonly saw amphipods without fairy shrimp than amphipods with fairy shrimp. Denton Belk did not support my speculation that amphipod predation could eliminate or prevent fairy shrimp colonization in alpine ponds of the Wind River Mountains.

Amphipods are usually swimming so fast they can’t be photographed but this one has stopped to try to eat a large ant (round, reddish-black head below the amphipod’s head). Such opportunistic predation suggests amphipods would eat fairy shrimp when available.

2 amphipods caught in the net at Box Lake. The larger one at right is partially curled up while the legs of the smaller one at left have bright reflections.
If predation by amphipods were a limiting factor in the abundance of ponds with fairy shrimp, most of the ponds with amphipods would not have fairy shrimp. That appears to be the case for the 366 pond visits where I made some effort to identify aquatic animals other than fairy shrimp. On 33 of those visits, I saw amphipods and on 12% of those visits I also saw fairy shrimp. In all 3 ponds where I saw both amphipods and fairy shrimp, I saw them at the same time. This suggests that fairy shrimp do not avoid potential amphipod predation by hatching earlier, as they may do for other predators. The lack of overlap between amphipod and fairy shrimp populations may be due to different environmental preferences rather than due to predation. For example, in Wyoming, I found amphipods but never fairy shrimp in some of the ponds associated with springs (e.g., Diamond Springs and MacLean Spring, Antelope Hills). However, that accounts for only 2 of the 33 amphipod sightings. On the other hand, Cliff Edge Pond (Wind River Mountains) is close to 4 smaller fairy shrimp ponds near “Coon Lake” but has amphipods without fairy shrimp (all 3 visits). In the northern Wind River Mountains, amphipod-bearing Jim Creek Pond is only about 450 m northwest of the similarly sized Jim Creek Saddle Pond, which has fairy shrimp. Other animals were not noticed in either pond. For those who want to calculate other statistics, I saw fairy shrimp on 180 visits and didn’t see fairy shrimp on 186 visits (N.B. this does not include visits where I did not make an effort to identify aquatic animals, often because I didn’t see fairy shrimp).
Predators of Fairy Shrimp – top
Copepod Predators
Copepods are a common and widely distributed subclass of the crustacean class Maxillopoda. One wouldn’t expect copepods to be predators of fairy shrimp due to their small sizes. They are best known as major consumers of phytoplankton in both marine and freshwater environments. The National Museum of Natural History says the “usual length of adults is 1-2 mm” but some “may be as long as 10 mm” (https://naturalhistory.si.edu/research/invertebrate-zoology/research/copepods). Nonetheless, in a microcosm experiment in an Arctic pond, the copepod Cyclops vernalis was found to prey on nauplii and juvenile fairy shrimp of the species Branchinecta paludosa and Polyartemiella hazeni (Figure 6-7, Stross and others, 1980). Such predation could explain the dramatic decrease in the density of fairy shrimp nauplii in Pond C from 2 per liter in mid-June 1971 to 0.3 per liter in mid-July (Stross and others, 1980, p. 294) without a concomitant increase in adult fairy shrimp (Stross and others, 1980, Figure 6-4, p. 256). The Cyclops vernalis population peaked at the end of June in 1971 (Stross and others, 1980, Figure 6-2, p. 254).
In Smith Creek Cold Springs Ponds Fairy Shrimp Video 2021-04-23r, you can see copepods swimming with fairy shrimp and cladocerans in a white bucket (Crowded Cohabitants of Smith Creek Cold Springs Ponds on the Fairy Shrimp Videos page). The copepods are the black specks.
Predators of Fairy Shrimp – top
Ostracod Predators
Ostracods are small swimming crustacean bivalves of the class Ostracoda. They live in both marine and terrestrial waters, including ephemeral ponds with low or high TDS. Some live in the same ponds as fairy shrimp. “Ostracods are generally tiny animals, with a body length mostly ranging from 0.2 mm to 2.0 mm.” (www.marinespecies.org/ostracoda/). The largest freshwater species can be 8 mm long (en.wikipedia.org/wiki/Ostracod).
Moore (1969, p. R178) listed “ostracodes (Cypris, Cyclocypris)” as predators of fairy shrimp. I couldn’t confirm this but I did find accounts of ostracods that eat other animals.
The predatory ostracod Australocypris insularis inhabits temporary, high-TDS salt pans with the fairy shrimp Parartemia cylindrifera in Australia (Campbell, 1995). A. insularis is a good swimmer capable of feeding on other swimming animals. Adult A. insularis are longer than 2 mm (Campbell, 1995). P. cylindrifera is a “large (males 22.3 mm), distinctive species” of fairy shrimp that occurs in waters with TDS of 3,000-140,000 mg/L (Timms, 2012). In microcosm experiments starting with egg-bearing soil from “Lake Cantara South” in South Australia, densities of copepods (Crustacea: class Maxillopoda, subclass Copepoda) of the genus Calamoecia and of the smaller ostracods of the genus Diacypris were negatively correlated with densities of A. insularis due, at least in part, to predation (Campbell, 1995). No conclusions regarding the eating of fairy shrimp could be drawn because only 1 individual of P. cylindrifera was observed among all the trials. Because the sampling interval was greater than 1 month, abundant P. cylindrifera could have hatched but been eaten as nauplii by the ostracods.
In a review of non-marine, giant ostracods, Rahman and others (2023) identified Australocypris insularis as a member of this group but did not state the size range of any giant species. They defined giant as greater than 3 mm long. “Australocypris species are important predators in salt-lake systems” and “A. insularis has been seen congregating on and consuming living and dead individuals of Parartemia brine shrimp (M. Rahman, unpubl. data)” (Rahman and others, 2023). According to Halse and McRae (2004), “Lacrimicypris kumbar is the largest ostracod known from inland Australian waters.” A. insularis must be less than the 5 mm length of L. kumbar adults.
The ostracod Heterocypris incongruens feeds on bacteria, algae, organic detritus, plant material and also on the cladoceran Daphnia magna (class Branchiopoda: order Anomopoda, family Daphniidae), copepods (Crustacea: class Maxillopoda, subclass Copepoda), chironomids (Insecta: order Diptera, family Chironomidae), other ostracods, round worms (phylum Annelida: class Clitellata, subclass Oligochaeta), insect larvae, and amphibians [eggs?] (Rossi and others, 2011). Field observations of ponds where H. incongruens had been newly introduced indicated high population densities of the ostracods within a few weeks. The ostracods commonly occurred in swarms with their shells touching in the afternoon (Rossi and others, 2011). In laboratory experiments, 100 H. incongruens individuals (less than 2 mm long) in a petri dish with 20 milliliters of pond water fed on the 1 chironomid larva (15 mm long) or 3 mosquito larvae offered. Chironomid and mosquito larvae are not strong swimmers. Swarms of ostracods typically formed around the prey, possibly due to the release of prey body fluids (Rossi and others, 2011). It took about 2 hours to consume a chironomid larva. When 10 clonal female ostracods were placed in the petri dishes, they were observed to eat each other (Rossi and others, 2011). It is not clear that the pond water contained sufficient alternate foods to provide food choices.
Predatory ostracods have been documented but ostracods seem like dubious predators of fairy shrimp due to the small sizes of most species, e.g., in East Stone Cabin Lower Reservoir. Also, see Win Wan Corral Pond Ostracod Video 2023-10-12r on the Clam Shrimp and Ostracod Videos page.
For more on ostracods, go to the Other Crustaceans You May Find With Fairy Shrimp page.
The examples of prey given above are half the size or smaller of juvenile and adult fairy shrimp or are poor swimmers. However, early hatching ostracods could eat fairy shrimp nauplii before they grow to be more than a millimeter or two long. The live Parartemia eaten by A. insularis may have been moribund rather than swimming normally. However at high densities, ostracods could form swarms capable of attacking and eating larger animals. Rossi and others (2011) cited examples from the literature of ostracod swarms feeding on “relatively quiescent” fish [probably marine], marine bristle worms (phylum Annelida: class Polychaeta), and masses of frog eggs. I’m surprised there hasn’t been a horror flick made of ravenous ostracod swarms attacking unsuspecting swimmers.
“Oh, look at the cute little swimming clams.”
“Aaaaaaah.”
Predators of Fairy Shrimp – top
Tadpole Shrimp Predators (Crustacea: class Branchiopoda, order Notostraca)
Like fairy shrimp, tadpole shrimp (order Notostraca) are branchiopods that have adapted to life in ephemeral ponds. Tadpole shrimp are shaped like a horseshoe crab with U-shaped carapaces covering their heads and thoraxes and with tails projecting to the rear (see Tadpole Shrimp Videos). They can be up to 100 mm long (Belk, 1982) but are usually less than 50 mm (EIGWUU). They spend most of their time feeding on pond bottoms and can churn up the sediment with vigorous leg movements.
Fryer’s (1988) lengthy descriptions of the legs of tadpole shrimp and their functions indicate that they differ from those of fairy shrimp in many respects and are well adapted for preying on small bottom-dwelling and swimming animals. Unlike other branchiopods, tadpole shrimp have mandibles that can be used for biting rather than just for grinding and crushing (Fryer, 1988, p. 28, 58). Tadpole shrimp are not filter feeders (Fryer, 1988, p. 29) although they can eat fine particles. They have many more taste receptors than other large branchiopods so they can consume a wider range of foods (Fryer, 1988, 54). The tadpole shrimp food groove is broader than that of fairy shrimp, clam shrimp, and some cladocerans (Fryer, 1988, p. 82). Adult tadpole shrimp “seize and devour small crustaceans and tiny chironomid larvae when these are supplied” (Fryer, 1988, p. 84), presumably in a laboratory setting. “[T]he endopodites can curl around active prey to prevent its escape” (Fryer, 1988, p. 84). Cases of cannibalism and eating the dead of their own species have been reported (Fryer, 1988, p. 80, citing Arnold, 1966). Tadpole shrimp are omnivorous. “As well as animal prey, flocculent detritus and particulate material, vegetable matter is also eaten” (Fryer, 1988, p. 89).
Kaestner (1970, p. 98) wrote that tadpole shrimp may feed on “amphibian eggs, weakened fairy shrimps, smaller tadpole shrimps, ostracods, cladocerans, soft insect larvae, and tadpoles”. Moore (1969) stated that tadpole shrimp “have been known to gnaw on” earthworms, mollusks, and dead tadpoles. Tadpole shrimp eat mosquito larvae and can be used to suppress mosquito populations (Su and Mulla, 2002, abstract only).
Microcosm experiments with crustaceans, insects, and invertebrates from playa lakes in Texas manipulated the presence of tadpole shrimp to see what effect that had on other species (Yee and others, 2005). Fairy shrimp of the genus Streptocephalidae hatched from 11-21% (over 4 trials) of the samples of 41 playa lake soils. Streptocephalid abundances were actually greater in the microcosms with natural hatches of tadpole shrimp than in those from which tadpole shrimp had been removed. Although the positive correlation between streptocephalid abundance and tadpole shrimp abundance was statistically significant, the details are not impressive. 28 or 173 streptocephalids occurred in trials with natural aundances of tadpole shrimp and 61 or 118 fairy shrimp in trials where tadpole shrimp were removed after hatching (Yee and others, 2005). Results are complicated by the presence of tadpole shrimp in the latter 2 trials that hatched after the initial removal. In any case, predation by tadpole shrimp appeared to be lacking.
I have found only 2 reports of tadpole shrimp definitively eating fairy shrimp. Citing another report, Bostoff and others (2010) stated that tadpole shrimp of the species Lepidurus lemmoni ate fairy shrimp of the species Branchinecta mackini in Mojave Desert ponds. Rogers and Timms (2017) stated that tadpole shrimp are predators of fairy shrimp as explanation for why they were removed from hatches used in their experiments.
I don’t doubt that tadpole shrimp are at least occasional consumers of fairy shrimp but the repeated occurrence of tadpole shrimp and fairy shrimp in the same pond is so common in the literature (e.g., Maeda-Martinez and others, 1997) and in my experience (e.g., 7 of 11 ponds with fairy shrimp in the “Burnt Lake” area of Middle Washoe County) that any effects of such predation are generally trivial.
Predators of Fairy Shrimp – top
Insect Predators Overview
Insects, either larvae, adults, or both, are important predators in ponds without fish. They were identified as predators, in general, of fairy shrimp by Moore (1969, p. R178), Moore (1963), Kaestner (1970, p. 90), and Pennak (1978, p. 337).
For context, Thorp and Covich (2001) provided general information about insects that inhabit freshwater aquatic environments of North America. They did not identify food sources of the various insect orders and families in sufficient detail to know for sure whether they eat fairy shrimp but they usually mentioned whether insect families included predators.
Reviewing insect predators that might eat fairy shrimp by order:
- Order Odonata (dragonflies, damselflies) larvae eat “mostly other insects” (Thorp and Covich, 2001, p. 669).
- Order Plecoptera (stoneflies) includes a few species with predacious larvae but they live in streams (Thorp and Covich, 2001, p. 672).
- Order Trichoptera (caddisflies) includes some species with predaceous larvae (Thorp and Covich, 2001, p. 676-680). Some species construct portable cases to live in, some construct nets of silk, but some are free-living (Thorp and Covich, 2001, p. 675) and more likely to be predators.
- Order Megaloptera (fishflies, alderflies) larvae are predators and “often attain a very large size” (Thorp and Covich, 2001, p. 680-681).
- Order Diptera (flies and midges) includes some families with predatory species, such as those in the families Chaoboridae and Chironomidae (Thorp and Covich, 2001, p. 692-698).
Of the order Hemiptera families:
- Suborder Gerromorpha (water striders and other walkers on water) “feed mostly on invertebrates of an appropriate size in the surface film” (Thorp and Covich, 2001, p. 682).
- Belostomatidae (giant water bugs) adults are “powerful predators” that can take fish and frogs (Thorp and Covich, 2001, p. 684).
- Corixidae (water boatmen) “Unlike other aquatic and semiaquatic Heteroptera, which are predators, corixid adults and nymphs feed primarily on detritus, algae, protozoans, and other extremely small animals. Adults of a few species, however, will capture and eat larger insects, such as mosquito larvae.” (Thorp and Covich, 2001, p. 684).
- Nepidae (water scorpions) “ambush invertebrates or small vertebrates” (Thorp and Covich, 2001, p. 684).
- Notonectidae (backswimmers) larvae and adults pursue or ambush “other invertebrates and small vertebrates” (Thorp and Covich, 2001, p. 685).
- Pleidae (pygmy backswimmers) adults and nymphs are less than 3 mm (0.1″) long and feed on “small invertebrates” (Thorp and Covich, 2001, p. 685).
Of the order Coleoptera families:
- For the Dytiscidae, the common name – predacious diving beetle – apparently says it all because Thorp and Covich (2001, p. 688) did not mention what they eat.
- Gyrinidae (whirligig beetles) larvae feed on other invertebrates (Thorp and Covich, 2001, p. 689).
- Noteridae (burrowing water beetles) “adults are mostly predators that live in close association with aquatic vegetation” (Thorp and Covich, 2001, p. 690).
- Hydrophilidae (water scavenger beetles) larvae are all predators (Thorp and Covich, 2001, p. 692).
Of the orders and families above, all but stoneflies (Plecoptera) could eat fairy shrimp. Stoneflies can’t because they are only found in streams. Insect predators listed below are discussed in more detail because I have been able to find additional information.
Dytiscid Predators (Hexapoda: class Insecta, order Coleoptera, family Dytiscidae)
Backswimmer Predators (Hexapoda: class Insecta, order Hemiptera, sub-order Heteroptera, family Notonectidae)
Water Boatmen Predators (Hexapoda: class Insecta, order Hemiptera, sub-order Heteroptera, family Corixidae)
Dragonfly and Damselfly Predators (Hexapoda: class Insecta, order Odonata)
Caddisfly Predators (Hexapoda: class Insecta, order Trichoptera)
Chaoborus Predators (Hexapoda: class Insecta, order Diptera, family Chaoboridae)
A few other families of insects are discussed very briefly in Other Insect Predators.
Predators of Fairy Shrimp – top
Dytiscid Predators (Hexapoda: class Insecta, order Coleoptera, family Dytiscidae)
Dytiscids are members of the family Dytiscidae (Insecta: order Coleoptera). A common name for them is predacious diving beetle. Moore (1963) mentioned Coleoptera predators and Pennak (1978, p. 337) specifically identified larvae of the family Dytiscidae. Both larvae and adults are well known consumers of fairy shrimp (e.g., Beladjal and Mertens, 2009).
Dytiscid larvae are easy to identify. They were probably the first insect predator I could identify in a pond. The abdomen tapers from a broad thorax and curves up over the body like a scorpion tail when the larva is standing in the water. Larvae have large, curved, pincer-like mandibles on each side of the relatively small head. Dytiscid larvae can be longer or shorter than fairy shrimp. Adult dytiscids are beetles that can swim and fly. They also eat fairy shrimp and other invertebrates. I can’t identify dytiscid adults so I use the term diving beetle for any unidentified (i.e., not a backswimmer or water boatman) beetle swimming in the water. If a pond is drying up or short on food, the adults can fly to other ponds.
I have seen dytiscids in several ponds with or without fairy shrimp.

Small, young dytiscid larva on a leaf. In this view, the head with antennae and pincers can be clearly seen but they are not fearsome yet. The pond had fairy shrimp at the time.

A dytiscid larva at center has a broad, pale abdomen, with forked tail-like features at the end that is curved up toward the water surface. Its head, on the pond bottom, is obscured by a small plant stem. The fairy shrimp do not appear to be avoiding the larva. That may be partly due to the high density of fairy shrimp and partly to the fact that the larva is not moving.
If predation by dytiscids were a limiting factor for the distribution of fairy shrimp, most of the ponds with dytiscids would not have fairy shrimp. The converse is true for the 366 pond visits where I made some effort to identify aquatic animals other than fairy shrimp. On 27 of those visits, I saw dytiscids and on 89% of those visits I also saw fairy shrimp. 88% of the time, I saw dytiscids and fairy shrimp at the same time. 12% of the time, the dytiscids were in ponds where I had seen fairy shrimp earlier in the year or in a different year. In fact, fairy shrimp may be a positive indicator for the presence of dytiscids. Although I saw dytiscids on only 7% of the 366 pond visits, the probability of finding dysticids was almost twice as high if the pond had fairy shrimp, 13%. For those who want to calculate other statistics, I saw fairy shrimp on 180 visits and didn’t see fairy shrimp on 186 visits (N.B. this does not include visits where I did not make an effort to identify aquatic animals, often because I didn’t see fairy shrimp).
Although they look nasty, dytiscids don’t seem to be a problem for fairy shrimp.
Back to Insect Predators List
Backswimmer Predators (Hexapoda: class Insecta, order Hemiptera, sub-order Heteroptera, family Notonectidae)
Backswimmers are members of the family Notonectidae (Insecta: order Hemiptera, suborder Heteroptera). They are commonly referred to as notonectids. Moore (1963) specifically identified backswimmers as predators of fairy shrimp.
Adult backswimmers are fast swimmers with beetle-like bodies generally shorter than most adult fairy shrimp. They have a pair of long, oar-like legs that ease identification. They swim on their backs and typically hang upside down from the water surface while at rest. The adults have piercing mouth parts that can kill a fairy shrimp and suck all the fluids out.
I have seen backswimmers in several ponds with or without fairy shrimp.

The backswimmer at center is swimming with its back characteristically down and its pair of long, oar-like legs angled forward. The body is a little less than 10 mm (0.4″) long. There are also several brown copepods in the photograph. The copepods may be too small to be of interest to the backswimmers or there may be so many copepods that there aren’t enough backswimmers to eat them all. Upper South Fork Pine Creek Pond Toquima Range does not have fairy shrimp.
Backswimmers and water boatmen look similar. Although backswimmers swim with their backs down and water boatmen swim with their backs up, it is not always easy to tell which side is up. Early on, I thought they had brown backs and pale stomachs (or whatever the other side is called) so if I saw brown it was a water boatman and if I saw pale it was a backswimmer. I have since seen backswimmers that are pale on both sides and water boatmen that have pale dirt on their backs. Color is not a reliable indicator. If you want to be able to tell the difference between a backswimmer and a water boatman, have a look at the video below. At least the ones I have seen can be distinguished by their swimming behaviors. As shown in Maul-Spuller Trail Pond Backswimmer Video 2024-08-20cr3, backswimmers often just hang motionless near the surface of the water. When you approach the pond and startle them, they swim rapidly away for short distances. They may try to hide under rocks. In contrast, water boatmen spend most of their time clinging to vegetation or anything else on the bottom of the pond. Their backs may collect dirt and make them hard to see. Water boatmen also swim rapidly away when startled but they stay close to the bottom of the pond. To compare the 2, watch Top of Dunderberg Creek Pond Water Boatmen Video 2024-09-10cr2 in the Water Boatmen Predators section below.
Maul-Spuller Trail Pond Backswimmer Video 2024-08-20cr3
If predation by backswimmers limited the distribution of fairy shrimp, most of the ponds with backswimmers would not have fairy shrimp. It’s a toss-up for the 366 pond visits where I made some effort to identify aquatic animals other than fairy shrimp. On 62 of those visits, I saw backswimmers and on 47% of those visits I also saw fairy shrimp. 83% of the time, I saw backswimmers and fairy shrimp at the same time. 17% of the time, the backswimmers were in ponds where I had seen fairy shrimp earlier in the year or in a different year. For those who want to calculate other statistics, I saw fairy shrimp on 180 visits and didn’t see fairy shrimp on 186 visits (N.B. this does not include visits where I did not make an effort to identify aquatic animals, often because I didn’t see fairy shrimp).
Backswimmers are not as common with fairy shrimp as dytiscids but also don’t have an exclusive relation with fairy shrimp.
Back to Insect Predators List
Water Boatmen Predators (Hexapoda: class Insecta, order Hemiptera, sub-order Heteroptera, family Corixidae)
Water boatmen are members of the family Corixidae (Insecta: order Hemiptera, suborder Heteroptera). They are commonly referred to as corixids. Water boatmen have been conclusively identified as predators of Artemia franciscana in “Great Salt Lake” by Wurtsbaugh and Berry (1990) and of Artemia parthenogenetica in southwestern Spain by Cespedes and others (2017).
Like backswimmers, adult water boatmen are fast swimmers with beetle-shaped bodies. The ones I have seen are most commonly 5-10 mm long. I haven’t seen any longer than 15 mm. Also like backswimmers, they have a pair of long oar-like legs. Unlike backswimmers, water boatmen swim back side up and cling to vegetation or debris at the bottom of a pond while at rest.
I have seen water boatmen in ponds with or without fairy shrimp.

This beetle has long legs for a rowing-like motion like backswimmers but it is a water boatman. It is darker colored than backswimmers and swims with its back up. Unfortunately, when it is hard to tell whether the legs are above or below the body and what color the animal is, whether due to waves or turbidity or molting, an accurate identification is iffy. The oar-like legs are not enough. Water boatmen eat fairy shrimp in some cases.
As discussed in the Backswimmer Predators section above, it may be hard to distinguish water boatmen from backswimmers. They look similar. However, the ones I have seen have different swimming behaviors. Top of Dunderberg Creek Pond Water Boatmen Video 2024-09-10cr2, below, shows typical water boatmen behavior. They stay close to the bottom of the pond and are hard to see if they collect mud on their backs. Maul-Spuller Trail Pond Backswimmer Video 2024-08-20cr3, above, shows the typical behavior of backswimmers for comparison.
Top of Dunderberg Creek Pond Water Boatmen Video 2024-09-10cr2
Because the quote from Thorp and Covich (2001) at the start of the Insects section suggests the diets of most corixid species are not dominantly of animals, it worth summarizing some of the contrary evidence. In his Ph.D. dissertation, Martin (1969) specifically rejected the beliefs that water boatmen feed primarily on detritus and plants. He found that water boatmen ate flies (Drosophila) placed on the water surface although not as readily as backswimmers did. Martin’s (1969) detailed descriptions of the heads and feeding structures of water boatmen showed that they are functionally similar to those of backswimmers although the details differ. They are used to pierce the prey, inject digestive enzymes, and then suck out the fluids. This feeding method is best adapted to animal prey although water boatmen also eat filamentous algae.
Feeding experiments with a wide range of diet items indicate that water boatmen prefer animals. Defining feeding as holding prey for at least 10 seconds, Reynolds (1975) found that 2 species of water boatmen from small lakes in British Columbia ate chironomids (Insecta: order Diptera, family Chironomidae) and daphnids (cladocerans, Branchiopoda: order Anomopoda, family Daphniidae) more than anything else. They also ate fairy shrimp. Dead and living diaptomids (Crustacea: class Maxillopoda, subclass Copepoda, order Calanoida, family Diaptomidae), damselflies (Insecta: order Odonata, suborder Zygoptera), chaoborids (Insecta: order Diptera, family Chaoboridae), amphipods (Crustacea: class Malacostraca, order Amphipoda), and mayflies (Insecta: order Ephemeroptera) were consumed as well. Consumption of mixed plankton, dead mixed plankton, macrophytes, and algae was minor. Reynolds (1975) further collected wild water boatmen of 6 species and used serological methods to check for 11 diet items in their guts. Only 1 species gave positive results for blue-green algae and macrophytes. The most common diet items across all species were chironomids and damselflies. In addition, daphnids and mayflies were popular with some species.
More recently, a species of water boatmen, Trichocorixa verticalis, was found in “Great Salt Lake” when high runoff raised lake levels to historic highs and lowered TDS to about 50,000 mg/L. Normal TDS of about 100,000 mg/L is too high for T. verticalis. The abundance of Artemia franciscana in the lake fell to historic lows as a result of predation by T. verticalis (Wurtsbaugh and Berry, 1990). The density of Artemia franciscana juveniles and adults was only 37 per cubic meter in 1986 compared to historical observations of 5,000-18,000 individuals per cubic meter. Artemia abundance declined after May while the abundance of water boatmen increased, peaking at 97 per cubic meter in September (Wurtsbaugh and Berry, 1990). Tellingly, the water boatman population collapsed to less than 5 per cubic meter in October without more A. franciscana to eat. As a follow-up, Wurtsbaugh (1992, abstract only) found that T. verticalis reduced Artemia nauplii densities from 103 per liter to 6 per liter in a microcosm experiment of 9 days.
The same dynamic is at play in the Odiel salt ponds of southwestern Spain. T. verticalis is invasive there. Although Artemia parthenogenetica is abundant in many of the ponds, “Artemia are low in abundance or absent in ponds of [TDS below 100,000 mg/L] where corixids are present” (Cespedes and others, 2017). Laboratory experiments with wild T. verticalis boatmen and A. parthenogenetica collected from a pond with TDS of 90,000 mg/L showed that the water boatmen, which were less than 5 mm long, ate more nauplii than juvenile and adult fairy shrimp. The more Artemia they were offered, the more the water boatmen ate. Cespedes and others (2017) also noted that T. verticalis ate cladocerans and chironomids.
The presence of water boatmen in high-TDS waters is potentially problematic for the long term future of Artemia and Parartemia. In the relatively simplified food web of “Great Salt Lake”, for example, they have a large impact on the Artemia population (Wurtsbaugh and Berry, 1990). If water boatmen species progressively adapt to more saline water over time, they may eventually invade the last bastion of fairy shrimp habitat free of aquatic predators, waters with more than 100,000 mg/L TDS.
An extensive summary by Hadicke and others (2017) of the reported feeding habits of water boatmen provided numerous examples of predation and of consumption of plants and material stirred up from detritus, which includes small animals. Food habits are likely to vary somewhat by species and by food availability. Some species are simply too small to pose much of a threat to fairy shrimp. Species of the family Micronectidae are generally less than 2 mm long. Those are not the water boatmen I have seen. I don’t think I could identify something that small on the pond bottom. One of the only clear cut conclusions of Hadicke and others (2017) is that species of the subfamily Cymatiainae “seem to be exclusively predators”. This would be helpful for those who can identify water boatmen to the subfamily level.
The data summarized above indicate that water boatmen can reduce fairy shrimp populations. Nonetheless, of the 58 pond visits when I saw water boatmen, I also saw fairy shrimp on 79% of those visits. 61% of the time, I saw water boatmen and fairy shrimp at the same time. 39% of the time, the water boatmen were in ponds where I had seen fairy shrimp earlier in the year or in a different year. For those who want to calculate other statistics, I saw fairy shrimp on 180 visits and didn’t see fairy shrimp on 186 visits (N.B. this does not include visits where I did not make an effort to identify aquatic animals, often because I didn’t see fairy shrimp).
As in the case of dytiscids and backswimmers, fairy shrimp populations I have visited commonly co-exist with water boatmen. The severity of water boatmen predation likely depends on the habitat and species.
Back to Insect Predators List
Dragonfly and Damselfly Predators (Hexapoda: class Insecta, order Odonata)
The order Odonata includes dragonflies (suborder or infraorder Anisoptera) and damselflies (suborder Zygoptera). Different species live in streams, ponds, and marshes. Some odonatans have eggs that enter diapause, like those of fairy shrimp (Thorp and Covich, 2001, p. 671). This allows them to persist in ephemeral ponds. The adults fly and eat other flying insects but the larvae are aquatic. Moore (1963) identified Odonata nymphs, or larvae, as predators. Species of a few families are known to stalk or ambush prey (Thorp and Covich, 2001, p. 670-671).

Exoskeleton molted from dragonfly larva floating on the surface of Marys River Peak North Pond, Jarbidge Mountains. This is probably a better view of the larva’s anatomy than a photograph of a live larva in the mud on the pond bottom. The 3 short pointed features at the end of the abdomen indicate this is a dragonfly larva rather than a damselfly larva. Damselfly larvae have 3 long feather-shaped gills at the ends of their abdomens instead (Kenney and Burne, 2000).

Adult damselfly perched on a rush stem in Marys River Peak Middle Pond, Jarbidge Mountains. The fact that it is holding its wings parallel to its body indicates it is a damselfly rather than a dragonfly.
Damselfly and dragonfly larvae are important predators in temporary ponds in the northeastern United States, at least (Kenney and Burne, 2000). Such ponds may have fairy shrimp. Damselfly larvae are large, are good swimmers, and “prey on invertebrates and small vertebrate animals” (Kenney and Burne, 2000). Different species of dragonfly larvae may stalk or they may ambush prey (Kenney and Burne, 2000). Sounds like bad news for fairy shrimp.
I have only rarely seen what I think are Odonata nymphs in the ponds I have visited but flying dragonflies or damselflies aren’t rare. Unfortunately, I noted Odonata nymphs or adults for only 19 of the 366 pond visits where I made some effort to identify aquatic animals other than fairy shrimp. On 12 of those visits I also saw fairy shrimp and on 7 I didn’t. Both were present on 9 of the 12 visits with fairy shrimp. These meager results don’t provide much insight into the significance of dragonfly and damselfly nymph predation on fairy shrimp. For those who want to calculate statistics, I saw fairy shrimp on 180 visits and didn’t see fairy shrimp on 186 visits (N.B. this does not include visits where I did not make an effort to identify aquatic animals, often because I didn’t see fairy shrimp).
Back to Insect Predators List
Caddisfly Predators (Hexapoda: class Insecta, order Trichoptera)
Johansen (1921, p. 23) wrote that “caddis-flies” eat fairy shrimp. Kaestner (1970, p. 90) stated that “caddis flies” eat fairy shrimp, perhaps as a repetition of Johansen (1921). Pennak (1978, p. 337) included “caddis larvae” in his list of enemies of “phyllopods”, by which he means large branchiopods (i.e., not cladocerans, which are small and eaten by everything).
Species of caddisflies (Insecta: order Trichoptera) may have markedly different lifestyles. Some larvae use silk to construct retreats or to filter food, such as algae, detritus, and macroinvertebrates (Thorp and Covich, 2001, p. 675), which could include fairy shrimp. Most, but not all, of the silk makers live in streams. Larvae of other species construct tubular cases with sand or vegetation debris and live in them. They are bottom-feeding “grazers” that don’t swim (Thorp and Covich, 2001, p. 754). There are also species that have “free-living” larvae which “are mostly predators on other arthropods” (Thorp and Covich, 2001, p. 675). Arthropods includes insects and crustaceans. 2 of the free-living larvae families with predatory species, Rhyacophilidae and Hydrobiosidae, however, live in streams and would be no threat to fairy shrimp.
Other Trichoptera families that have at least some species which are predaceous and may live in ponds according to Thorp and Covich (2001, p. 676-682) are:
- Polycentropodidae (“most species are predators”),
- Leptoceridae (“some are primarily predators and few feed exclusively on freshwater sponges”),
- Limnephilidae (“larvae of one species are known to be carnivores”),
- Molannidae (“feed on . . . even invertebrates”), and
- Phryganeidae (“large”, “mostly predators”).
There must be caddisfly larvae eating fairy shrimp somewhere but the only caddisfly larvae I can identify are the ones that live in tubular cases that they have constructed. The trichopteran engineers are generally less than 20 mm long and crawl along pond bottoms with their heads barely poking out of their homes of glued sand or bits of vegetation. They can’t swim. That is obvious when they fall off a steep rock and drift slowly to the bottom of the pond. Such larvae could eat fairy shrimp eggs but not swimming fairy shrimp.

This is a caddisfly larva in a case made of bits of twig. Its head is at the left end of the case, where one protruding leg can be seen. The case looks too cumbersome for these larvae to be a threat to swimming fairy shrimp.

The 4 pale, tubular objects are cases of caddisfly larvae which have been constructed of sand grains. The larvae are actively climbing up the rocks and occasionally rolling down so they can climb up again. Do they look like they could eat fairy shrimp?
I haven’t compiled data on how often I have seen caddisfly larvae with and without fairy shrimp because the only caddisfly larvae I can identify are the case builders, which I think are harmless.
Back to Insect Predators List
Chaoborus Predators (Hexapoda: class Insecta, order Diptera, family Chaoboridae)
Species of the genus Chaoborus are commonly referred to as phantom midges and adults have a similar size and appearance as mosquitoes (Salmela and others, 2021, p. 68). However, they have shorter mouth parts and the females don’t feed on blood. The larvae are “voracious” consumers of rotifers, cladocerans (Crustacea: class Branchiopoda, orders Anomopoda, Ctenopoda, Onychopoda, Haplopoda), copepods (Crustacea: class Maxillopoda, subclass Copepoda), and mosquito larvae (Salmela and others, 2021, p. 69). They also eat chironomid larvae (Insecta: order Diptera, family Chironomidae) and ostracods (Crustacea: class Ostracoda) (Borkent, 1979, p. 192). Chaoborus larvae use their antennae to grasp prey and can swim rapidly (Salmela and others, 2021, p. 68). In lakes without fish, they can be the dominant predator.
That sounds threatening enough for fairy shrimp but Chaoborus larvae are small. They are thin cylinders 4-20 mm long (Borkent, 1979, p. 136) and the small gap between their mandibles (probably less than 2 mm) limits what they can eat (see the excellent Figure 5, p. 90, of Salmela and others, 2021). The prey items mentioned above all have maximum dimensions less than 5 mm. Nonetheless, predation by Chaoborus nyblaei larvae caused “major population declines” of Branchinecta paludosa in alpine southern Norway (Lindholm and others, 2016b, abstract only). How they did that is not explained in the abstract, of course, but a “major population decline” warrants further consideration.
C. nyblaei has also been found in northern Norway, Sweden, and Finland. In Finland, adults produce 1 clutch of eggs per year, the eggs are resistant to drying and freezing in the winter, and the larvae hatch in the spring (Salmela and others, 2021, p. 110), like fairy shrimp. Most other Chaoborus species do not produce resistant eggs and survive the winter as larvae in the unfrozen water or mud of permanent ponds. That C. nyblaei survives in temporary ponds makes it a greater threat to fairy shrimp. After hatching, the larvae reach lengths of 18-22 mm and the adults appear in Finland in late June through August (Salmela and others, 2021, p. 110). These Finnish larvae are known to eat other chaoborids (Salmela and others, 2021, p. 110). This makes Lindholm and others’ (2016b, abstract only) claim more convincing.
If C. nyblaei is chewing up fairy shrimp in Fennoscandia, other fairy shrimp populations are probably at risk. Chaoborus nyblaei is a member of the subgenus Schadonophasma. This subgenus has the largest adults and larvae of the Chaoboridae family (Borkent, 1977, p. 127) and includes C. trivittatus and C. cooki (Borkent, 1977, p. 195). Those 2 species occur in northern North America and in parts of California (Borkent, 1977, Figures 16 and 18, p. 231, 233). Larvae are 11-20 mm long (Borkent, 1977, p. 136). C. cooki has eggs that survive winters in temporary ponds (p. 147) but C. trivittatus does not (Borkent, 1977, p. 140). Given the morphological and life cycle similarity of C. cooki to C. nyblaei, it would likely eat fairy shrimp if available. Predation by C. trivittatus is also possible but less certain.
UV radiation may limit the Schadonophasma risk in higher elevation ponds. In alpine southern Norway, C. nyblaei preferentially occupied colored ponds with high concentrations of dissolved organic carbon at lower elevations rather than clear ponds at higher elevations (Lindholm and others, 2016b, abstract only). This is because C. nyblaei suffers much higher rates of DNA damage due to UV radiation in clear water than in brown water. Although the abstract of Lindholm and others (2016b, abstract only) did not report elevations, the abstract of Lindholm and others (2012, abstract only) considered Branchinecta paludosa sites at elevations of 1,500 m (4,920′) “high alpine”. Water browning was attributed to higher temperatures enabling more plant growth (and decay) at the lower elevations. On the other hand, Lamontagne and others (1994) found a few clear lakes in the Canadian Rocky Mountains with Chaoborus trivittatus (or possibly C. cooki) at elevations of 1,600-2,250 m (5,250-7,380′).
Fittingly, fairy shrimp-hungry chaoborids have been eliminated from the Sierra Nevada by the stocking of another fairy shrimp predator, fish (Stoddard, 1987; Knapp and Marine Science Institute, 1996). In Canada, populations of C. trivittatus and the smaller C. americanus were drastically reduced in the summer following autumn fish-stocking of cutthroat trout (1 lake) or dolly varden (1 lake) in the University of British Columbia Research Forest and were effectively eliminated within 2 years (Northcote and others, 1978). Chaoborids’ large body sizes relative to other zooplankton make them preferred prey for fish. For more on fish-stocking zooicide, have a look at the East-Central Sierra Nevada or the Wind River Mountains pages.
Back to Insect Predators List
Other Insect Predators
I haven’t found and haven’t looked very hard for information on other families of predaceous insects identified by Thorp and Covich (2001). An excellent resource for fairy shrimp habitats in the northeastern United States is A Field Guide to the Animals of Vernal Pools by Kenney and Burne (2000). It has excellent photographs and short descriptions and can be viewed at https://vernalpool.org. Vernal pools are off-stream, ephemeral ponds that fill from precipitation in the winter and spring. Fairy shrimp occur in some of them. If the Vernal Pool Guide lists a particular family of predaceous insect, it’s reasonable to assume it eats fairy shrimp unless the larvae or adults are too small. Vernal pools are only 1 of many fairy shrimp habitats but I haven’t come across anything comparable for alpine or desert ponds, rock pools, or prairie potholes.
Order Megaloptera (fishflies, alderflies) – Kenney and Burne (2000) identified at least 1 family that lives in fairy shrimp habitat. Those fishfly larvae are up to 50 mm (2″) long and have pinching mandibles to “subdue aquatic insects and other invertebrate prey” (Kenney and Burne, 2000).
Order Coleoptera:
Family Hydrophilidae (water scavenger beetles) – Adults are omnivores but larvae “hide in vegetation to await prey” and are up to 38 mm (1.5″) long (Kenney and Burne, 2000).
Family Gyrinidae (whirligig beetles) – Fairy shrimp are probably safe from the adults, which are omnivores that feed at the water surface, but the larvae “are predaceous” and are up to 38 mm (1.5″) long (Kenney and Burne, 2000).
Family Noteridae (burrowing water beetles) – These were not mentioned in Kenney and Burne (2000).
Order Hemiptera:
Family Belostomatidae (giant water bugs) – Giant water bugs in the northeastern United States are up to 64 mm (2.5″) long. They are bad news for fairy shrimp. Giant water bugs “kill anything they can” (Kenney and Burne, 2000) and have been nicknamed fish killers and toe-biters.
Family Nepidae (water scorpions) – Kenney and Burne (2000) confirmed that water scorpions live in fairy shrimp habitat in the northeastern United States, are as large as or larger than fairy shrimp, and are ambush predators which eat small vertebrates and invertebrates, which could include fairy shrimp.
Family Derrida (water striders) – Water striders are predators that live in fairy shrimp habitat but they hunt only at the water surface (Kenney and Burne, 2000). If the larvae do too, fairy shrimp don’t have to worry.
Family Pleidae (pygmy backswimmers) – These were not mentioned in Kenney and Burne (2000).
Predators of Fairy Shrimp – top
Turbellarian Flatworm Predators (phylum Platyhelminthes: class Turbellaria)
As if insects weren’t enough of a problem, fairy shrimp are also eaten by some flatworms. Turbellarian flatworms of the genus Mesostoma live in some of the same ephemeral rock pools in Botswana that are inhabited by fairy shrimp of the species Branchipodopsis wolfi. In feeding experiments, turbellarians with average lengths of 3 mm mostly ate 1-day old nauplii with average lengths of 0.7 mm and 3-day-old nauplii with average lengths of 1.2 mm rather than longer 5-day-old juveniles or adults (De Roeck and others, 2005).
Attacks by the turbellarians were observed under the microscope. They would strike the front of a fairy shrimp as it swam by in an attempt to paralyze it. If that was successful, the turbellarian would attach itself to the fairy shrimp and suck out its fluid contents over a period of a few minutes (De Roeck and others, 2005). On the bright side, De Roeck and others (2005) found that the turbellarians did not eat fairy shrimp resting eggs, thus limiting the potential impact of turbellarians on the persistence of a rock pool population. Thorp and Covich (2001, p. 160-161) also discussed the predatory habits of Mesostoma and reported that one species attacks cladocerans and insect larvae.
Predators of Fairy Shrimp – top
Bird Predators
Birds are important predators of fairy shrimp even though Kaestner (1970) and Pennak (1978) did not mention them. Stilts and avocets “can be significant consumers” of fairy shrimp of the family Artemiidae in Australia (Timms, 2012). Alaska Ecology Cards (available online), produced by the Alaska Division of Wildlife Conservation, listed ducks and phalaropes in addition to water shrews and “diving beetles” as predators of fairy shrimp. Van Stappen (1996) reported that waterfowl, “especially flamingos” “are the most important natural dispersion vectors for Artemia fairy shrimp”. Redshank and blacktailed godwit at Castro Marim, Portugal, and Cadiz Bay, Spain, were found to be carrying eggs of Artemia franciscana and A. parthenogenetica (Green and others, 2005, abstract only).
Fairy shrimp are a minor component of the diets of some duck species. Esophagus contents of laying female ducks captured in the prairie potholes region of North Dakota were 14% fairy shrimp for northern pintail (n=31), 6% for northern shoveler (n=15), 5% for blue-winged teal (n=20), 4% for mallard (n=37), and less than 1% for gadwall (n=55) (Swanson, 1984).
Artemia franciscana are recognized as an important food source for waterfowl at “Great Salt Lake” (Utah Department of Environmental Quality, 2011). The Gilbert Bay food web (Figure 9) indicates that A. franciscana is a major food source for California gulls, eared grebes, northern shovelers, phalaropes, and goldeneyes. More than 50% of the food consumed by northern shovelers and green-winged teals overwintering at Great Salt Lake was resting eggs of A. franciscana in 2004-2006. About 20% of the northern shovelers’ food was adult fairy shrimp (Vest and Conover, 2010).
Eared grebes at “Great Salt Lake” in the autumn feed primarily on Artemia franciscana. They need to consume about 28,000 individuals per day to meet their nutritional needs for survival and to continue their migration (Conover and Caudell, 2009). Conover and Caudell (2009) cited an earlier study by Cooper and others (1984) that estimated eared grebes eat 8,000-70,000 A. franciscana per day. Eared grebes catch their prey individually with their bills and they must do so at a rate of about 2 A. franciscana per second while they are underwater if they are to put on enough fat for their migrations (Conover and Caudell, 2009).
Analysis of the stomach contents of birds at “Mono Lake” found that 92% was Artemia monica in 13 California gull chicks, 7% in 5 Wilson’s phalaropes, and 4% in 21 northern phalaropes (Winkler, 1977, Fig. 5-3, p. 96). Jones and Stokes Associates (1993c) reported A. monica individuals account for 90% of the diet of eared grebes at “Mono Lake” in late summer (p. 3F-23) and an unknown percentage of the diet of Wilson’s phalaropes (p. 3F-31). A. monica was 57% of the diet of California gull chicks in another study reviewed by Jones and Stokes Associates (1993a, p. C-13).
Ackerman and others (2014) considered the species Artemia franciscana a prey item for migratory American avocets and black-necked stilts at San Francisco Bay but did not quantify its importance.
The populations of Artemia parthenogenetica in the Odiel and Tinto estuaries in southwestern Spain have high levels of infection by a parasite which uses flamingos as the final host (Sanchez and others, 2016).
If you are not interested in math, skip the following digression on conditional probability (Skip).
Simple field observations of birds in fairy shrimp ponds strongly suggest that birds eat fairy shrimp. Wading avocets and some ducks drag their bills back and forth through the water. Phalaropes peck at the water while swimming or, less commonly, while wading along the shore. Although correlation is not cause, I saw suggestive associations at Wyoming ponds between avocets, phalaropes, and fairy shrimp.
Antelope Hills:
- avocets observed at 13 ponds, 8 of those had fairy shrimp
- phalaropes observed at 9 ponds, 4 of those had fairy shrimp
Granite Mountains:
- avocets observed at 6 ponds, 5 of those had fairy shrimp
- phalaropes observed at 6 ponds, 5 of those had fairy shrimp
Great Divide Basin:
- avocets observed at 8 ponds, 3 of those had fairy shrimp
- phalaropes observed at 3 ponds, 1 of those had fairy shrimp
Looking at the conditional probabilities for a single pond visit rather than for ponds (as above), the probability of finding fairy shrimp was 47% for visits to ponds with water in the Antelope Hills, Granite Mountains, and Great Divide Basin (51 out of 108 pond visits). That is not counting rock pools because I never saw birds at rock pools. Avocets improve your chances. The probability of finding fairy shrimp given that an avocet is present during the visit is 63%. Conversely, the probability of finding fairy shrimp if no avocet is present is 41%. But look anyway.
[19 visits had fairy shrimp and avocets (true positives) and 11 had avocets but no fairy shrimp (false positives). 46 visits had no fairy shrimp and no avocets (true negatives) but 32 had fairy shrimp and no avocets (false negatives).
probability of fairy shrimp, given avocets = probability of fairy shrimp with avocets / probability of avocets = 19/30, where the observed frequency is the estimate of probability]
These data are biased by my occasional failures to record data for ponds when I did not find fairy shrimp but there are more serious deficiencies. I could argue that the true conditional probabilities for avocets is better than what I calculated because I did not spend the whole day at a pond waiting for avocets and did not take into consideration avocets close by, such as avocets at “Scotty Lake” but not at North “Scotty Lake” West Pond when it had fairy shrimp or avocets at Eastern “Soda Lake” but not at “Soda Lakes” Far Eastern Pond when it had fairy shrimp or avocets at “Piaya Lake” but not at Little “Piaya Lake” when both had fairy shrimp. Alternatively, I could argue that the true conditional probabilities for avocets are worse than what I calculated because the avocets at Northeastern “Lewiston Lakes”, Cuesta Pond, and Chinook Pond were really there for the clam shrimp and not for the fairy shrimp. Or, maybe it is more about nesting preferences than food sources.
For phalaropes, it is close to a coin toss. The probability of finding fairy shrimp given that a phalarope is present during the visit is 53%. Conversely, the probability of finding fairy shrimp if no phalarope is present is 46%. The more important point is that it is worth keeping track of the clues.
[10 visits had fairy shrimp and phalaropes (true positives) and 9 had phalaropes but no fairy shrimp (false positives). 48 visits had no fairy shrimp and no phalaropes (true negatives) but 41 had fairy shrimp and no phalaropes (false negatives).
probability of fairy shrimp, given phalaropes = probability of fairy shrimp with phalaropes / probability of phalaropes = 10/19, where the observed frequency is the estimate of probability]

One phalarope is on the water to the right of center and 2 are along the shore (only the rear end of the one at left is visible). “Coyote Lake” Antelope Hills has fairy shrimp.

Several avocets wading and a few more are coming in for a landing (at right). Although avocets feed on fairy shrimp where available, they also help disperse the eggs. Separation Rim “Soda Lake” Great Divide Basin has fairy shrimp.

The ducks behind the fence post at center are standing in Labou North Playa Pond Fairview Valley which currently has fairy shrimp and apparently nothing else. These and other ducks have been dragging their bills back and forth through water less than 5 cm (2″) deep in what appears to be feeding behavior.

The birds floating on and standing in the water include ducks and maybe coots and the 2 birds with white markings at left are probably gulls. Rhodes Big Lake (Rhodes Salt Marsh) has fairy shrimp. Gulls at “Mono Lake”, 90 km (54 miles) to the west, eat lots of fairy shrimp (see above).
There are many more photographs of birds and fairy shrimp in the chronicles.
Predators of Fairy Shrimp – top
Fairy Shrimp Predators (class Branchiopoda: order Anostraca)
Fairy shrimp inhabit 2 niches that have resisted colonization by all but a few predators. One such niche is opaque, clay-rich water that has low to moderate TDS and alkaline pH. The opaque water is not conducive to large phytoplankton populations but fairy shrimp nonetheless seem to find enough to eat. Predatory dytiscids (e.g., Beauty Peak East Pond, June 12, 2019, Bodie Hills) and water boatmen (e.g., Playa Wire Gate Pond, Soda Spring Valley and Stinking Springs Well Pond, Rawhide Flats) were able to make the jump to opaque water but amphibians and most insect predators could not. As in clear water, fairy shrimp populations have been able to co-exist with dytiscids and water boatmen in opaque water. The insect predators evidently leave lots of adult fairy shrimp uneaten.
All those uneaten fairy shrimp present an opportunity for other predators if they can only figure out how to live and hunt in opaque water. Fairy shrimp had already adapted to opaque water so it was a short step to evolution of a giant fairy shrimp that could eat other fairy shrimp. 2 species of giant, predatory fairy shrimp have been identified in North America and both live only in opaque water. They are Branchinecta gigas (e.g., Belk, 1975 and 1982) and Branchinecta raptor (Rogers and others, 2006). The 2 species may have evolved independently as they are not genetically close (Rogers and Aquilar, 2020).
The predatory fairy shrimp of North America look very similar to other fairy shrimp except that they are more than 50 mm long (Edwards Creek Valley) at full size and the morphology of the legs and their bristles differ in detail. Their eyes are also smaller relative to body size than those of other species. B. gigas individuals from the Mojave Desert were observed to eat Branchinecta mackini smaller than 15 mm whole and to nip larger ones in two (Brown and Carpelan, 1971). They could eat 35 B. mackini in a day. Most of the 50% decline in the abundance of B. mackini in an Alberta lake over a 3-week period in May was attributed to B. gigas predation by Daborn (1977). According to Fryer (1966, abstract only), the stout spines on the legs of the predators do not allow filter feeding. Nonetheless, “[c]opepods, fairy shrimp eggs, and organic detritus have also been found in the guts of predatory fairy shrimp” (Kaestner, 1970, p. 92). Daborn (1977) observed B. gigas eating copepods (Crustacea: class Maxillopoda, subclass Copepoda) and cladocerans (Branchiopoda: orders Anomopoda, Ctenopoda, Onychopoda, and Haplopoda) in addition to fairy shrimp.
The Australian species Branchinella occidentalis has been identified as predatory (Rogers and Timms, 2017) but it is not quite so far along in its adaptation to a predatory lifestyle as the North American species. Maximum lengths are less. Individuals only become predatory when they reach their largest sizes of about 50 mm. The eyes of B. occidentalis are reduced like those of the North American predators and the mouth parts are modified in similar ways but the legs lack adaptations for a predatory lifestyle (Rogers and Timms, 2017). The habitat of B. occidentalis was described as “turbid” by Rogers and Timms (2017) whereas B. gigas waters are opaque. That begs the questions of whether B. occidentalis could also live in clear water and what role insect predator competition has had in controlling its evolution.
Branchinella occidentalis was not identified as predatory when it was first described. However, when biologists finally looked, the evidence for predation was easy to find. Large individuals of Branchinella occidentalis were observed to eat other species of fairy shrimp less than about 20 mm long (Rogers and Timms, 2017). Live fairy shrimp were consumed abdomen first. Dead fairy shrimp were ignored. B. occidentalis uses touch to identify prey but it does not exhibit the same hunting methods as the North American species. B. occidentalis also eats filamentous green algae, non-floating plants, diatoms, chironomid larvae (Insecta: order Diptera, family Chironomidae), and cladocerans (Rogers and Timms, 2017). It remains to be seen whether B. occidentalis will become more like the North American species over the next few hundred thousand years or evolve in a different direction.
Branchinecta ferox becomes “carnivorous” as it matures (Fryer, 1983, abstract only). Its length can exceed 45 mm. It eats copepods and cladocerans (Fryer, 1983, abstract only) but I haven’t found a reference that says it specifically eats fairy shrimp. B. ferox lives in Europe and Asia (Lindholm and others, 2016a).
The other niche with few predators is clear, high-TDS water. This is where brine shrimp of the genera Artemia and Parartemia live. Certain predatory species of water boatmen have evolved to tolerate TDS greater than 50 mg/L but not yet greater than about 100 mg/L. As yet, there are no giant, predatory Artemia or Parartemia but who knows where evolution may lead? Maybe the energy cost of osmoregulation prevents the evolution of a giant species in high-TDS waters, or maybe not.
Predators of Fairy Shrimp – top
Human Predators
Humans are also fairy shrimp predators. Artemia franciscana from “Great Salt Lake” were used as food by local Native Americans (Pennak, 1978, p. 337). Southern Paiutes feasted on alkali fly (previously referred to as brine fly) larvae at “Mono Lake” but also ate Artemia monica (www.newworldencyclopedia.org/entry/Paiute). Now, humans collect adults or eggs of the genus Artemia from salty lakes and salt works throughout the world for use as fish and shellfish food.
Artemia eggs (marketed as cysts) are sold to those who feed the nauplii (hatchlings) to juvenile fish or shellfish larvae. In the larviculture of marine fish and shellfish, Artemia nauplii “constitute the most widely used food item” (Van Stappen, 1996). Artemia eggs have thus become a hot commodity with over 2,000* metric tons (2,200 U.S. tons) sold annually worldwide as of 1996 (Van Stappen, 1996). This may be an underestimate. The harvest from “Great Salt Lake” alone from 1989 through 1997 was 2,680-6,600 metric tons annually but that is the wet weight (Lavens and Sorgeloos, 2000). For a quick look at the breadth of the industry, scroll through search results for “Artemia cysts”. Originally (1950s), the eggs came from “Great Salt Lake” but now they are also collected from salt works in San Francisco Bay, China, Siberia, and elsewhere. Artemia have been introduced into salt works in South and Central America, Australia, and Southeast Asia (Van Stappen, 1996). It seems likely that they have been introduced into natural lakes, too, but Van Stappen (1996) did not make that clear. Fairy shrimp evidently do not interfere with salt production. In fact, they may improve salt quality by eating up the phytoplankton.
*A variety of vendors claim a range of 200,000-300,000 Artemia nauplii per gram of cysts and high hatching rates. Hatching rates are less than 90% (as low as 70% for some products) so each gram actually has more eggs than the number of nauplii that are expected to emerge. Assuming 200,000 eggs per gram for a conservative estimate, 2,000 metric tons (2 x 10^9 grams) would have about 4 x 10^14, or 400 trillion, eggs. Just a kilogram (2.2 pounds) would have about 200,000,000 eggs. Do humans kill more than 400 trillion individuals of any other animal genus every year?
In Thailand, the freshwater fairy shrimp Streptocephalus sirindhornae is used as food for ornamental fish and prawns (Rogers and others, 2013b). Adults are also collected from the wild and eaten by people in northeastern Thailand (Rogers and others, 2013b, citing Sanoamuang and Dumont, 2000).
High variability in the nutritional characteristics of Artemia nauplii have caused problems for the larviculture industry. Bio-encapsulation methods have been developed to add unsaturated fatty acids, vitamins, chemotherapeutics, and vaccines (Van Stappen, 1996). This is apparently accomplished by adding these products to the water and capitalizing on the non-selective filter feeding and maybe absorption by the nauplii.
Earth now has gazillions more Artemia fairy shrimp than ever before. Most are fed to fish or shellfish larvae, which anostracans evolved specifically to avoid, within a few days of hatching. Something to ponder the next time you come upon a high-TDS pond with a bunch of fairy shrimp or eat farmed “seafood”.
Humans also kill Artemia for toxicology studies. Since 1975, the Artemia Research Center at the State University of Ghent, Belgium, has developed and promoted the use of Artemia toxicology tests for marine and other high-TDS environments (Personne and Wells, 1987). By then, cladocerans (Branchiopoda: order Anomopoda) of the family Daphniidae had been adopted as routine test animals for assessing toxicity in freshwater environments. Why not use another branchiopod, Artemia, for salty environments? After all, Artemia nauplii can be reliably hatched from eggs within 24 hours and there is no need to wait for them to grow up (Personne and Wells, 1987).
Comparisons of the responses of daphnids and Artemia to the same pollutants or effluents were used to better sell the Artemia text kits. Daphnid test animals have to be taken from a maintained laboratory culture whereas Artemia can be hatched from dried eggs without all that extra maintenance expense. The Artemia tests can be completed more quickly, too (Personne and Wells, 1987). Sold! Whether for routine monitoring, screening before ocean dumping, lethal toxicity determination, ranking chemicals by toxicity, testing the toxicity of mixtures, sublethal bioassay, or maybe even teratogenic assay (Personne and Wells, 1987), the Artemia Research Center recommends killing Artemia nauplii. There are numerous Artemia toxicity test kits advertised online.
Predators of Fairy Shrimp – top
The Artemia Research Center is still at it. Sections in Dhont and Sorgeloos (2002) include “Beneficial Effect of Artemia in Salt Production”, “Artemia as Instant Live Food”, “Use of Juvenile and Adult Artemia”, “Valorisation of Organic Waste and Use of Artemia in Effluent Treatment”, “Artemia in Ecotoxicology”, “Artemia as a Didactic Tool”, and “Artemia in Personal, Social, and Sex Education”.
A freshwater fairy shrimp species has also been condemned to toxicology tests. The user guide for Thamnotoxkit F, “Crustacean Toxicity Screening Test for Freshwater” by Microbiotests, a Belgian company, can be found online. Thamnotoxkit F is an implementation of the ISO 14380:2011 standard for “Water quality – Determination of the acute toxicity to Thamnocephalus platyurus (Crustacea, Anostraca)”.
The user guide, or “Standard Operating Procedure”, for Thamnotoxkit F is informative and well written. The procedure is pretty simple. Prepare a solution of “Standard Freshwater” by adding the contents of 5 vials of solutions with high concentrations of ions such as calcium and sulfate to deionized water. Add “Standard Freshwater” and more deionized water to a test tube with eggs, shake it, and pour it into a petri dish. Incubate the petri dish under constant illumination for 20-22 hours. Add various concentrations of the possible toxin, effluent, wastewater, etc., to small depressions on a test plate. Under a microscope, add 10 T. platyurus nauplii to each test well using a micropipette. After 24 hours, count how many nauplii are moving and how many are not moving. The goal is to determine the concentration that kills 50% of the animals, or LC50. LC50 is a standard measure of toxicity that is used by regulatory agencies to regulate chemicals, compounds, and effluents. Results are more reliable if the test wells exhibit a range of mortality from 100% to 0%.
It may sound cruel to kill off 50% or more of newly hatched nauplii to achieve a measure of toxicity that is not easily translated into human risk. However, those that aren’t killed by the tested chemical or effluent are probably just flushed down the sewer or left to dry up on the test plate. No testing lab is in the business of raising fairy shrimp. It’s safe to say that none of the eggs in any Thamnotoxkit F or any Artemia test kit ever grow to be mature fairy shrimp. So add the eggs for toxicology tests to the 400 trillion killed as fish or shellfish food each year.
A more benign use of Artemia eggs has been for sale as Sea Monkeys. They come with a book-size plastic aquarium, a salt packet, and a food packet. I tried this. The fairy shrimp can live for a few weeks until the food packet runs out. I didn’t know what else fairy shrimp could eat at the time. I have since learned that yeast can be used as food if you can’t grow your own algae culture. The tiny Sea Monkey aquarium looks awfully cramped. The transparent aquarium walls could also be a problem. Easy Chair Crater Fairy Shrimp Video 2023-04-13a-cr and Squaw Flat Playa Lake Fairy Shrimp Video 2023-04-16-cr (videos) show that fairy shrimp sometimes swim head on into the white walls of a container. They may have similar difficulties avoiding transparent walls. Better to get out of the house and search for fairy shrimp in the wild. It’s a lot more fun for you and for them.
Predators of Fairy Shrimp – top