Tadpole Shrimp (class Branchiopoda: order Notostraca)
Clam Shrimp (class Branchiopoda: Conchostraca or orders Spinicaudata, Laevicaudata, Cyclestherida)
Ostracods (class Ostracoda)
Cladocerans (class Branchiopoda: orders Anomopoda, Ctenopoda, Onychopoda, Haplopoda)
Copepods (class Maxillopoda, subclass Copepoda)
If you go searching for fairy shrimp, there will likely be times when you don’t find any. Do not despair, there is more to ponds than fairy shrimp. Fish-free ponds have an abundance of animal species other than fairy shrimp and some of them are visible. Thorp and Covich (2001) wrote a book on freshwater invertebrates that is 1,056 pages long, and that is only for North America. Below, I try to provide enough information and photographs to allow you to recognize tadpole shrimp, clam shrimp, ostracods, cladocerans, and copepods. There are also lots of bird, insect, mollusk, and other species you could find but I have to draw the line somewhere.
Tadpole Shrimp (class Branchiopoda: order Notostraca)
Tadpole shrimp are branchiopods of the subclass Calmanostraca, order Notostraca. They are not likely to be confused with anything else. Their bodies are 20-100 mm (0.8-3.9″) long (Belk, 1982). They look like little horseshoe crabs, in spite of the common name. They have a flexible, variably translucent, shield-like carapace covering the head and most of the body. It is commonly colored pale to dark brown and may be mottled. Near the front of the carapace are 2 black, kidney-shaped compound eyes that are close together. Between them and slightly toward the rear is a dark nuchal organ (Pennak, 1978) or dorsal organ (Moore, 1969) that may have the same origin as the ocellus of fairy shrimp. The thorax and forward abdominal segments have more than 35 pairs of legs but they don’t stick out from underneath the carapace. Oddly, there may be 2 or more pairs of legs on a tadpole shrimp “segment” (Pennak, 1978, p. 328). The rearward abdominal segments lack legs and are not covered by the carapace. The last abdominal segment is called the telson. There are 2 long, thin, segmented, tail-like features that extend from the telson at the end of the body and are conspicuous. In some species, the telson also has a short, stubby supra-anal plate between the 2 “tails” (Belk, 1982). The genus Lepidurus has supra-anal plates and the genus Triops does not. The antennae are short and inconspicuous. The first (or first 2) pair(s) of legs are long and look like antennae. They have thin, whip-like branches that generally stick out laterally from under the front of the carapace. (Kaestner, 1970, p. 95-97; Pennak, 1978, p. 328-329). The first pair of legs has a sensory role and probes the pond bottom in front of and beneath the animal (Fryer, 1988).
Tadpole shrimp are denser than water. They generally walk along the pond bottom and may dig or burrow shallow depressions (Fryer, 1988). They occasionally swim and can swim as fast as fairy shrimp.
Tadpole shrimp are omnivores. They have more taste receptors than fairy shrimp or clam shrimp and eat a wider variety of foods (Fryer, 1988). They feed on the pond bottom eating small animals, organic particles, and vegetation. They have been reported to eat other tadpole shrimp (Fryer, 1988) and fairy shrimp (Tadpole Shrimp Predators). Fryer (1988) fed them Bemax (a breakfast cereal), hard boiled yolks, pieces of cooked meat, and cladocerans. He found they won’t eat gelatin with acetic acid and strongly reject worms soaked in quinine.
The eggs of tadpole shrimp, like those of fairy shrimp, survive desiccation, high temperatures, and freezing (Fryer, 1988). They may be fertilized sexually or hermaphroditically. They are carried by females or hermaphrodites in pouches on the 11th pair of legs until laid on the pond bottom or attached to vegetation (Fryer, 1988). The eggs remain viable for many years.
Tadpole shrimp are most commonly found in ephemeral ponds. They can also be found in temporarily flooded fields such as rice fields. Lepidurus arcticus can live in permanent water bodies and co-exist with fish (Fryer, 1988).

Tadpole shrimp, clam shrimp, and fairy shrimp on the white lid of a peanut butter jar. A whip-like branch of a front leg can be seen on the tadpole shrimp turned sideways at left. The tadpole shrimp at center has a supra-anal plate between its “tails”. Denton Belk identified samples from this lake as Lepidurus lemmoni.
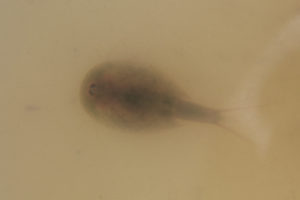
View down through murky water at a tadpole shrimp in a white container. The eyes on the carapace and the 2 “tails” at the end of the abdomen are easy to see. The long, whip-like branches of the first legs can be faintly made out above the carapace and, with difficulty, below.

Ventral view of a tadpole shrimp that flipped over in the net. It obviously has lots of legs but good luck counting them. The pale supra-anal plate can be seen at the end of the abdomen indicating this individual is a member of the genus Lepidurus.

A tadpole shrimp on playa mud with very little water. The sensory first pair of legs have left tracks in the mud to the sides of the carapace.

An inverted tadpole shrimp on playa mud. The red legs suggest high hemoglobin content due to oxygen stress (Guadagnoli and others, 2005, p. 3550). This tadpole shrimp is probably not in trouble as it can push against the mud with its abdomen to flip itself over. It is probably of the genus Triops because it does not have a supra-anal plate at the end of its abdomen.

A large tadpole shrimp in the net. Note the distinctive configuration of the 2 elongate compound eyes and the dark dorsal organ between them.

A tadpole shrimp swimming at the surface of opaque water. Tadpole shrimp don’t filter feed so maybe it is just having a look around.
Visit the tadpole shrimp videos page to see tadpole shrimp in action.
Clam Shrimp (class Branchiopoda: formerly order Conchostraca, now divided into orders Spinicaudata, Laevicaudata, and Cyclestherida)
Clam shrimp really do look like small clams, but they swim. Clam shrimp carapaces are up to 20 mm (0.8″) long (Belk, 1982) and may be translucent or colored from pale yellow to dark brown or red. In most species, the carapace is calcified (Kaestner, 1970, p. 100). There is not much visible detail. The bodies are flattened laterally within the carapace. The 10-32 pairs of legs (Kaestner, 1970, p. 99) are leaf-like and decrease in size toward the rear of the animal (Belk, 1982). They do not protrude from between the valves. The first one or two pairs may be used for clasping the female carapace during mating (Belk, 1982). Most clam shrimp have 2 close-set compound eyes but Cyclestheria has a single fused eye (Thorp and Covich, 2001, p. 891). The eyes are hidden by the carapace. Clam shrimp have 2 pairs of antennae. The first are small. The second are long and used for swimming, burrowing, and clinging (Belk, 1982). The end of a clam shrimp body has a pair of stout anal spines (Pennak, 1978, p. 328) but they are generally not visible. Depending on species, concentric growth lines may be seen on the valves.
Clam shrimp are more likely to be found near the pond bottom or in vegetation rather than swimming in open water. They can swim as fast as fairy shrimp. For feeding, water is drawn into the carapace and filtered for algae, rotifers, and other small animals by the legs (Thorp and Covich, 2001, p. 898). The clam shrimp in Bald Mountain Dry VABM Saddle Pond were seen digging into the pond bottom and possibly scraping plant stems whereas a probably different species in Kibby Flat Playa Lake was observed swimming at the surface of opaque water far from shore and presumably filter-feeding.
Clam shrimp can reproduce sexually, hermaphroditically (haploid eggs fertilized by same individual), or parthenogenetically (diploid eggs not fertilized) (Belk, 1982). 1 species of Spinicaudata may produce live eggs that hatch directly to nauplii but all the others apparently produce only resting eggs (Thorp and Covich, 2001, p. 894) that survive drying, heating, and freezing. Eggs are released into the pond when the mother molts (Kaestner, 1970, p. 101).
Identification of clam shrimp is complicated by ostracods, which are also swimming bivalves. Ostracods are smaller (see below) but there may be some overlap. If it’s a freshwater swimming bivalve and it’s 5-20 mm (0.2-0.8″) long, it’s probably a clam shrimp. If you thought the clams with legs in the B.C. comic strip or the strolling oysters in Lewis Carroll’s “Through the Looking Glass” (poem recited by Tweedledee) were a surprising novelty, wait until you see clam shrimp.
The small, brown, oval animals in photograph Northeastern “Lewiston Lake” 1993-06-10, #0424c, in the tadpole shrimp section above, are clam shrimp. Denton Belk identified clam shrimp from this lake as Caenestheriella belfragei.

A clam shrimp swimming near the pond bottom among sparse vegetation.

A clam shrimp swimming in a white container with some pond detritus. The antennae used for swimming can be seen protruding from the left end of the bivalve.

2 clam shrimp (Conchostraca) stuck together in water in a white container. They are mating. I inadvertently caught them in the act, which may last for several minutes.

A calm shrimp in the net with several fairy shrimp and a dytiscid larva (top). The small, brown clam shrimp is below and to the right of center.
Visit the clam shrimp videos page to see different clam shrimp feeding strategies in a mountain pond and in a playa pond.
Ostracods (class Ostracoda)
Ostracods (ostracodes in Thorp and Covich, 2001) are swimming or walking bivalved animals with rigid shells. The length of the shell can be as short as 0.2 mm (0.008″) (Thorp and Covich, 2001, p. 830) or as long as 2.5 mm (0.09″) (Thorp and Covich, 2001, p. 836). The length is significantly longer than the width, which is greater than the thickness. The hinged side is commonly convex and the other side straight or slightly concave (Thorp and Covich, 2001, p. 835-841). The shells commonly have colors of green, dirty white, yellow, pale brown, or reddish brown (Thorp and Covich, 2001, p. 827). The ostracod body is quite unlike that of branchiopods. It is not well segmented and consists of a head with 2 pairs of antennae, a thorax with 2 or 3 pairs of legs, and no abdomen. The antennae are used for swimming and walking and the legs for feeding, creeping, and cleaning out the interior of the shell (Thorp and Covich, 2001, p. 813). Ostracods have a single, small, median eye that can determine light intensity through the shell (Thorp and Covich, 2001, p. 816).
Ostracods swim with a sustained motion because if they don’t, they sink (Thorp and Covich, 2001, p. 827). They can swim faster than fairy shrimp and as fast as water boatmen. Although Thorp and Covich (2001, p. 827) write that ostracods “only swim short distances (usually between plants)”, the ones I have seen often swim more than a meter (3′) until I can’t see them anymore. In comparison, backswimmers and water boatmen generally swim less than that before stopping.
Ostracods mostly live and forage close to, on, or in pond bottoms but a few swim up into open water. Some don’t swim at all but crawl along the pond bottom or dig into the sediment for food. Algae and particulate organic matter are the most common ostracod foods but they also eat diatoms, tree pollen, and Paramecium. Some delve into carnivory, eating snail flesh, bee carcasses, or smaller ostracods (Thorp and Covich, 2002, p. 827). For more on the predatory tendencies of ostracods, see ostracods on the Predators page.
Freshwater ostracods reproduce either sexually or parthenogenetically but Thorp and Covich (2001, p. 817) go on to write that all ostracods are diploid and on the next page give examples of polyploidy. Whatever the case, eggs may either hatch quickly or enter diapause. Diapaused resting eggs survive drying and freezing and may remain viable for decades. Mothers carry eggs in the rear parts of their shells and may release them singly or in masses in selected parts of their habitats (Thorp and Covich, 2001, p. 817).
Resting eggs aren’t the only means for ostracods to survive the drying of temporary ponds. Ostracod nauplii can survive in moist, or even frozen, soil by entering a state of torpidity (Thorp and Covich, 2001, p. 826).
Ostracods inhabit “nearly every conceivable [terrestrial] aquatic habitat” (Thorp and Covich, 2001, p. 820) and also marine environments. The list includes ditches, caves, aquifers, organic mats in fens, and waters with TDS up to 170,000 mg/L (Thorp and Covich, 2001, p. 820). This suggests that ostracods are also very widely distributed.
Identification of ostracods is complicated by those other pond-dwelling swimming bivalves, clam shrimp. In my inadequate experience, there isn’t much to distinguish them from clam shrimp visually – just smaller and faster. I hope the photographs below help.

Ostracods and cladocerans less than 2 mm long at the surface of the water after being released from the net. The ostracods are the brownish-green, closed bivalves. The cladocerans are translucent, pale gray, oval animals with black eyes. Some have a black feature (possibly eggs) extending down their backs.

These ostracods at the edge of the water aren’t swimming much so they are easy to photograph. They are red and egg-shaped and barely 1 mm long. There is 1 at center, 2 to the left, and 1 at lower right.

Abundant tiny red ostracods on the mud and the ice at the edge of Win Wan Corral Pond in early December. Most of them may be dead but they could have switched to a torpid state to survive the cold. Some in the water are still moving. I saw ostracods again 3 months later but don’t know if those were winter survivors or new hatches.

Ostracod shell on dried mud in June. I last saw a live ostracod in April but the water lasted into May. Ostracod shells don’t decay easily so even if you get to a pond after it has dried up, it is worth checking out the dried mud for possible remains. Thanks to their shells, their ubiquity, and their relatively rapid morphological changes, ostracods are among the most important fossils for determining the ages of sedimentary rocks deposited in oceans and lakes.
Visit the clam shrimp videos page to see how ostracods get along in a stock pond on Win Wan Flat.
Cladocerans (class Branchiopoda: orders Anomopoda, Ctenopoda, Onychopoda, Haplopoda)
Cladocerans are close to the limit of what one can identify visually without a microscope. They are small, 0.2-18 mm (0.08-0.7″) long (Belk, 1982). Most are less than 3 mm (0.12″) long (Belk, 1982). In cross-section, they have a discus shape (Thorp and Covich, 2001, p. 851) and generally swim with the shorter dimension horizontal. They are round, ovoid, pear-shaped, or lumpy when viewed from the side and one end is rather pointed in some species (Thorp and Covich, 2001, p. 876-890). They have a carapace covering all but the head and a single compound eye (Dexter, 1959, p. 559). The carapace may be too transparent and the eye too small to see. The carapace is folded along the animal’s back (Belk, 1982) so it looks like a bivalve shell but it isn’t. Cladocerans aren’t clearly segmented (Thorp and Covich, 2001, p. 851) like fairy shrimp and tadpole shrimp. Within the carapace, there is a head; a thorax with 4-6 pairs of flat, leaf-like legs; and an abdomen without legs (Thorp and Covich, p. 851, 854). The legs are used for filter-feeding, handling food, scraping, creeping, and grasping females (Thorp and Covich, 2001, p. 854). Like other branchiopods, cladocerans have 2 pairs of antennae. The first is small and sensory and the second is long and used for swimming (Thorp and Covich, 2002, p. 851).
Cladocerans that spend most of their time swimming in open water eat mostly algae. Others seek algae, bacteria, and other organic matter on pond bottoms or scrape plant stems and leaves (Thorp and Covich, 2001, p. 862). Due to their small sizes, cladocerans could consume only the tiniest animals. The post-abdominal claw allows cladocerans to dislodge and reject a mass of food due to “taste” (Thorp and Covich, 2001, p. 854, quotation marks in original).
Cladocerans reproduce either sexually or, more commonly, parthenogenetically. The choice can be in response to environmental conditions (Thorp and Covich, 2001, p. 856). Some populations are entirely female. Parthenogenetic (diploid) eggs can either develop immediately into offspring or become resting eggs with diapausing embryos. Haploid eggs are fertilized by males and become resting eggs with diapausing embryos that survive drying, heating and freezing (Thorp and Covich, 2001, p. 856). Eggs are carried and develop in the space between the mother’s back and the carapace. Small species may carry only 2 eggs while large species may carry dozens. Eggs are released into the pond when the mother molts (Thorp and Covich, 2001, p. 857).
Cladocerans inhabit a wide diversity of freshwater habitats, including permanent lakes, ephemeral ponds, slow-moving streams, and near-shore vegetation of fast-moving streams (Thorp and Covich, 2001, p. 860) and rarely occur in marine habitats (Belk, 1982). Some live in the pore spaces of river gravels (Thorp and Covich, 2001, p. 860).
With cladocerans, the best I can hope for is to distinguish them from copepods, which are also small and rather common in ephemeral ponds. In that light, the best distinguishing feature of a cladoceran is the pair of branched second antennae that extend laterally from the front, or top, of the animal and are used for swimming (good figures in Thorp and Covich, 2001, p. 852, 876, 886, 888, 889, 890). Copepods also have a pair of antennae that extend laterally from the front of the body and are used for swimming but they are not branched (figures in Thorp and Covich, 2001, p. 919, 922). Because the cladoceran head is not covered by the fatter carapace, one rule of thumb that may be helpful in distinguishing cladocerans from copepods is that if the front end (as revealed by forward motion) is narrower than the back, then it is a cladoceran. Copepods generally have the fatter end at the front.
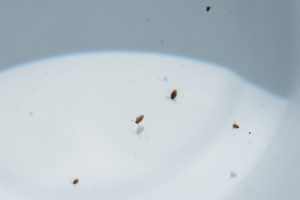
These cladocerans swimming in a white container have obvious brown shells covering all but the head, discoid shapes, and swimming appendages that are branched, particularly visible on the cladoceran at lower right. They are between 2 and 5 mm (0.08-0.2″) long but I didn’t make a good estimate of length at the time.

Cladocerans and fairy shrimp swimming in a white container. The animal at upper right has branched swimming antennae and is narrower at the front than the rear so it is almost certainly a cladoceran. The carapace is essentially transparent. I don’t know what the dark feature under the carapace is. It may be eggs. At the time, I estimated these cladocerans to be 2-3 mm (0.08-0.1″) long. The reddish brown swimmer at top center is probably a copepod even though it is almost as big as the cladocerans. In a different photograph, there is one of these with unbranched swimming appendages on the fatter front end of its body.
I don’t have many photographs of cladocerans. The first of the ostracod photos above, East Stone Cabin Lower Reservoir 2023 #10, also has cladocerans. 1 of the copepod photos below, South Sister Southwest Pond 2022 #19c, also has what I think are cladocerans.
Copepods (class Maxillopoda, subclass Copepoda)
Swimming freshwater copepods are mostly 0.5-2.0 mm (0.02-0.08″) long but some species are up to 5 mm (0.2″) long . Copepods have a segmented exoskeleton but no carapace. 5 or 6 pairs of legs stick out laterally from the body but they are too small to see. You might have better luck seeing the 2 tail-like appendages (rami) that extend from the rear end of the animal (Thorp and Covich, 2001, p. 915-916). I haven’t seen these either. Unlike cladocerans, copepods are approximately cylindrical in cross-section. Many species have a distinct change in thickness near the middle of the body with the front half fatter than the rear half (e.g., Thorp and Covich, 2001, Fig. 2 on p. 916 and Fig. 5 on p. 922). There is one feature that is a dead giveaway but it is not always present. When female copepods have eggs, the eggs fill 2 oblong pouches that stick out from both sides of the the rear third of the body (e.g., Thorp and Covich, 2001, Fig. 5 on p. 922). Visually, it looks like the rear of the body has a bulge or is just fat. Cladocerans don’t have anything like this. Copepods have a single, simple eye (Thorp and Covich, 2001, p. 915), which is inconspicuous.
The great diversity of the Copepoda subclass encompasses omnivores, detritivores, herbivores, and carnivores. Collectively, they eat algae, cyanobacteria, pollen, detritus, bacteria, rotifers, protozoans, fungi, crustaceans, chironomids (Insecta: order Diptera, family Chironomidae), chaoborids (Insecta: order Diptera, family Chaoboridae) and other Diptera larvae, and fish larvae (Thorp and Covich, 2001, p. 927-928).
All but a few species of copepods reproduce sexually (Thorp and Covich, 2001, p. 918). Most eggs hatch within a few days. Others enter diapause and become resting eggs that survive drying and freezing. Such eggs may remain viable for decades. Immature copepods, known as copepodids or copepodites, can enter diapause due to adverse environmental conditions, such as overcrowding or changing hours of daylight, and can also survive drying (Thorp and Covich, 2001, p. 921).
Copepods are common in oceans, lakes, wetlands, wet soils, and groundwater and can reach very high densities in their habitats. Their life styles range from free-swimming plankton in open water to parasites of fish (Thorp and Covich, 2001, p. 915-916).
The copepods you see while looking for fairy shrimp are likely to be smaller than the cladocerans you see but unless you see both in the same pond that doesn’t help much. As discussed above under Cladocerans, my preferred criterion for distinguishing copepods from cladocerans is that copepods use an unbranched pair of antennae for swimming and cladocerans use a branched pair of antennae for swimming. Unfortunately, it is difficult to see the antennae, much less whether they are branched. Body shape can help. Copepods are generally fatter at the front whereas cladocerans are generally fatter in the middle or the rear.
Both copepods and cladocerans swim with a jerky motion of short spurts in 1 direction and then another spurt in a different direction. Of those I have seen, copepods’ swimming was more start-stop and cladocerans’ was more continuous (shorter pauses) but I doubt that that is always true. Some copepods crawl on the bottom rather than swim (Thorp and Covich, 2001, p. 926). You probably won’t see those.

Abundant, unusually large, dark brown copepods at the edge of the water. They are 2 mm or more long and possibly the largest I have ever seen. They are unlikely to be cladocerans because they are fatter at the front.

The 2 animals at right have bulbous masses near their rear ends. I think these are egg pouches. If they are, these are copepods. The copepods at upper left and lower center do not have these masses but they could be males. Unbranched swimming antennae can also be seen.

Dark red copepods in South Sister Southwest Pond. Note the 2 unbranched swimming antennae at the top of the head of the animal near the top. The vertically oriented copepod below center has symmetrical bulges near the end of its abdomen which are likely the characteristic copepod egg sacs. There are 2 translucent animals with black interiors at lower right and near the bottom which have shapes different than the copepods. They may be cladocerans even though cladocerans are commonly larger than copepods.
Below, the cropped enlargements of photograph Smith Creek Cold Springs Ponds 2021-04-23, #09, show that making tentative identifications of cladocerans and copepods based just on size and shape is feasible and is right in some cases.

Animals from Smith Creek Cold Springs Ponds have been transferred to a white container for better viewing. There are a few fairy shrimp at left. The floating rush stem is 30 mm (1.2″) long. There are abundant, lenticular animals that are 3-4 mm (.12-.16″) long and look sort of like rice grains. Some are narrower at the front than at the rear so they are probably cladocerans. There are also a few dark specks that are only 1-1.25 mm (0.04-0.05″) long. Little detail can be seen at this scale but size alone suggests they are copepods.

This is a cropped version of photograph Smith Creek Cold Springs Ponds 2021-04-23, #09. Additional details can be seen in the suspected cladocerans. Most have a dark feature in the forward half of the body. It may be food in the digestive system. The shapes of the 2 suspected copepods in this view (above and to right of center, left of center) can now be discerned. The one to the left of center is fatter at the front. The one to the right of center has a dumbbell-like shape that is fat at both ends. This is likely a copepod with eggs.

This is the cropped lower right part of photograph Smith Creek Cold Springs Ponds 2021-04-23, #09c. Branched swimming antennae and a single black eye on a narrow head can be seen on one of the 2 suspected cladocerans at lower left. In the suspected cladoceran to the right of center, the segmented appearance of the rear half of the body can be resolved into 3 rows of 4 spherical eggs each. The fatter front end of the suspected copepod above center is more visible. At even greater magnification, a pair of unbranched swimming antennae can be seen extending laterally from the head. This confirms it is a copepod.