High concentrations of various major and trace elements in some samples of drain waters upstream of Stillwater National Wildlife Refuge and of lakes within the refuge prompted U.S. Fish and Wildlife Service biologists to test whether these waters were toxic to test species such as the cladoceran Daphnia magna, fathead minnow larvae, bluegills, and striped bass. Some of the tested waters killed all of the test animals within a few days.
Dwyer and others (1992, p. 513) found that water from “Pintail Bay” in the northern part of Stillwater National Wildlife Refuge was “acutely toxic” to aquatic animals and noticed that the relative proportions of major ions in the water were quite different from seawater, which has lots of animals. They suspected that might help explain the toxicity independently of potentially harmful trace element concentrations. The lower hardness, higher alkalinity, and higher sulfate concentrations of the “Pintail Bay” water were of particular interest. To test their hypotheses, they prepared solutions from laboratory salts to approximate most major ion proportions and the overall TDS of “Pintail Bay” water but at different hardness levels by manipulating calcium and magnesium concentrations. As a control, they prepared a seawater-like solution with about the same TDS as “Pintail Bay” from the commercial product Instant Ocean.
A note on hardness: Hardness is calculated by multiplying calcium and magnesium concentrations by the molecular weight of calcium carbonate, dividing by their respective atomic weights, and then adding the two. It is used to describe an aesthetic property of water. People don’t like very hard water so they pay to add chemicals to soften the water and the amounts to add are typically linked to hardness rather than to calcium and magnesium concentrations. Aquatic biologists also prefer to refer to hardness rather than to calcium and magnesium concentrations for reasons only they know. Some of the U.S. Environmental Protection Agency’s aquatic life criteria for specific trace elements are specified by exponential equations that use hardness as the variable (e.g., cadmium, nickel). Most scientific mentions of hardness specify “hardness (as CaCO3)”. CaCO3 is the formula for calcium carbonate and for the minerals calcite and aragonite. There is also something called non-carbonate hardness but I haven’t seen it used. The following graphs show concentrations of calcium and magnesium rather than hardness because they are more informative.
A note on alkalinity: Alkalinity is a measure that summarizes the acid-buffering capacity of a water. It is the sum of the concentrations of the dissolved bases that can neutralize acid. Between pH 6.5 and 10, bicarbonate is the dominant component of alkalinity in most fresh waters. Hydroxyl, phosphate, and borate anions can also contribute to alkalinity. Above about pH 10, the carbonate ion increases in concentration by the breakdown of bicarbonate and becomes the dominant component of alkalinity. In comparing the biologic suitability of natural waters, referring to bicarbonate concentration would probably be just as useful as referring to alkalinity. In dealing with acid effluents, alkalinity may be more helpful. Alkalinity is not calculated by summing the concentrations of the bases. It is measured in the lab by adding dilute acid slowly to a water sample until a specified pH is reached but not exceeded. The amount of acid added is then used to calculate alkalinity. The following graphs use alkalinity because the concentrations of bicarbonate were not reported in some studies. For other graphs and tables that used bicarbonate, just assume that the bicarbonate concentration is the same as the alkalinity.
Low and medium hardness solutions and Instant Ocean water killed 100% of Daphnia magna within 48 hours even when diluted to 70%, 49%, and 34% (Dwyer and others, 1992, Table 5, p. 517). 20% died in high hardness solution diluted to 34%. The TDS of the 34% dilutions is near the upper tolerance limit of Daphnia magna (Dwyer and others, 1992, Table 5, p. 518). D. magna is nonetheless appropriate because it is a widely distributed species and has been found in drift samples of the drains in the area (Hallock and others, 1993, p. 44). That it may have been killed by high TDS alone is a good measure of how degraded the waters at Stillwater Marsh are.
Striped bass live in fresh, brackish, and marine water and can tolerate TDS concentrations greater than those of the test solutions (Dwyer and others, 1992, p. 518). They appear to be sensitive to hardness though. The undiluted low and medium hardness waters killed all the striped bass (Dwyer and others, 1992, Table 6, p. 517). Undiluted high hardness water and Instant Ocean water didn’t kill any. The major ion concentrations of these and “Pintail Bay” waters are summarized in the table of Major Ions of Solutions Used in Striped Bass Toxicity Tests by Dwyer and Others (1992). When medium hardness water was diluted to 70%, none of the fish died. The results confirm that some Stillwater National Wildlife Refuge waters are more toxic than seawater-like solutions of the same TDS.
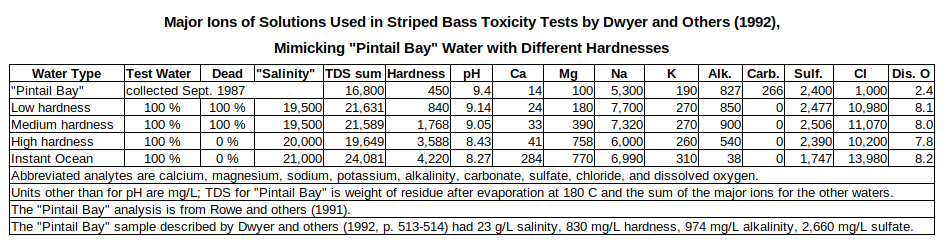
In the table of Major Ions of Solutions Used in Striped Bass Toxicity Tests by Dwyer and others (1992), the concentration of chloride in “Pintail Bay” water collected in September 1987 is probably closer to 7,000 mg/L than to the reported 1,000 mg/L because the measured TDS is much greater than the sum of the major ions. With 7,000 mg/L chloride, the sum of the major ions would be 16,565 mg/L rather than 10,565 mg/L. A March 1988 sample of “Pintail Bay” had a TDS of 12,200 mg/L, a sum of the major ions of 12,870 mg/L, and 5,000 mg/L chloride (Rowe and others, 1991).
Toxicity of Newlands Drain Water – top
The major ion chemistry of Stillwater National Wildlife Refuge waters and upstream drains is bad enough to kill but the trace elements make it worse. Dwyer and others (1992) added laboratory salts with arsenic, boron, copper, lithium, molybdenum, and strontium to the solutions of different hardnesses and Instant Ocean. Nominal concentrations were 0.8 mg/L arsenic, 36.1 boron, 0.04 copper, 0.93 lithium, 0.3 molybdenum, and 2.42 strontium. Actual concentrations were close to these values except for arsenic, which appeared to be less than 0.8 mg/L. Existing amounts of boron and lithium in Instant Ocean raised the boron concentration to 38 mg/L and lithium to 6.87 mg/L in the Instant-Ocean water with added trace elements. The addition of trace elements shifted 100% and 70% high hardness waters and 70% medium hardness water from harmless to completely lethal. At 50% dilution, medium hardness water with trace elements was 40% lethal rather than harmless. None of the striped bass died in Instant Ocean waters with added trace elements. Additional experiments by Dwyer and others (1992) further showed that the trace element concentrations in the test solutions were far below lethal concentrations individually. The lethal effects were due to some combination of the trace elements and their interactions with the major ions and each other.
The fact that diluted low, medium, and high hardness solutions with or without trace elements were less lethal than undiluted solutions complicates causal interpretations. Dilution gives apparently lethal low hardness solutions even lower hardness, which should be bad. Maybe that was somehow offset by beneficial effects of lower TDS, lower sulfate, lower pH, lower alkalinity, or something else in the solutions without trace elements.
For real-time toxicity tests, Finger and others (1993) exposed Daphnia magna, fathead minnow larvae, and bluegills to drain and lake waters in and upstream of Stillwater National Wildlife Refuge. Fathead minnows are relevant test animals as they have been collected from “Lead Lake” and “Dry Lake” in Stillwater National Wildlife Refuge. The tests were run for 10 days and the test waters were renewed with drain or lake samples collected daily. The 7 tested waters had large differences in concentrations. For example, ranges were 28-230 mg/L for calcium, 10-290 mg/L magnesium, 45-4,800 for chloride mg/L, 0.02-0.13 for arsenic mg/L, and 0.6-20.0 mg/L for boron.
Water from Hunter Drain killed all the bluegills and daphnids by the end of 3 days and all the fathead minnow larvae by the end of 5 days (Finger and others, 1993, Table 10, p. 30). It also killed 100% of saltwater-adapted mysid shrimp and 100% of sheephead minnow larvae within 6 days. “Lead Lake” wasn’t quite as toxic. Its waters killed 80% of daphnids, 60% of fathead minnow larvae, and 60% of bluegills after 9 days (Finger and others, 1993, Table 12, p. 32). “Stillwater Point Reservoir”, also within the refuge, was considerably less toxic with death rates of 25% for daphnids and fathead minnow larvae and 20% for bluegills after 9 days (Finger and others, 1993, Table 14, p. 34). Paiute Diversion Drain and D-Line Canal didn’t kill any of the test animals within 9 days (Finger and others, 1993, p. 25).
To compare the results across many elements, drain or lake water concentrations were normalized to (divided by) concentrations in “Lead Lake” water diluted to 50%. 50% “Lead Lake” water had a fathead minnow mortality rate of 30%. For waters with lower mortality rates, one would expect concentrations of potentially harmful solutes to plot below 1.0. For waters with higher mortality rates, one would expect concentrations above 1.0.
“Dilution” refers only to trace elements as “concentrations of constituents such as calcium, magnesium, sodium, potassium, chloride, and sulfate were maintained for all treatments”, as was pH, by manipulating concentrations of the dilution water (Finger and others, 1993). The following graphs use the median concentrations of the 9 daily samples for each site. This obscures some dramatic day to day changes. TDS (calculated as the sum of major ions) for Hunter Drain ranged from 550 to 25,650 mg/L over the 9-day test period.
Toxicity of Newlands Drain Water – top
As shown in the graph of Fathead Minnow Toxicity Profiles for Drains Upstream of and Within Stillwater National Wildlife Refuge, August 1987, the mortality expectations generally hold true. There is good separation of the more toxic 50% Hunter Drain, 50% TJ Drain, and 50% “Lead Lake” from the less toxic 50% “Stillwater Point Reservoir”, D-Line Canal, and Paiute Drain for TDS, boron, molybdenum, lithium, sulfate, and strontium although in some cases 50% “Stillwater Point Reservoir” has lower enrichments than harmless D-Line Canal and Paiute Diversion Drain or 50% TJ Drain has higher enrichments than the more toxic Hunter Drain. Selenium is also potentially toxic except that 50% “Lead Lake” water has a lower concentration than the 3 less toxic waters. The rankings of arsenic enrichments have such a poor correlation with death rates that arsenic is unlikely to be a significant cause of the animal deaths. No case can be made that zinc has a toxic effect. The rankings are consistent with higher alkalinity and lower oxygen concentrations being associated with mortality but the differences between toxic and non-toxic concentrations are not as great as for the trace elements.
Data from Finger and others (1993).
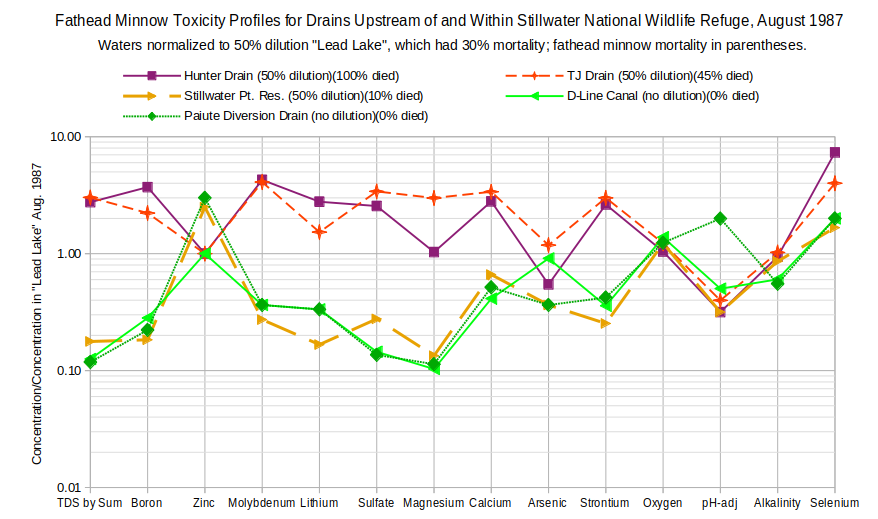
The good separation of more toxic and less toxic waters also holds for magnesium and calcium but any affect could be subsumed within that of TDS. In contrast to the work of Dwyer and others (1992), higher magnesium and calcium concentrations and, hence hardness, are associated with higher death rates. Undiluted D-Line Canal water didn’t kill any daphnids, fathead minnow larvae, or bluegills in Finger and others’ (1993) experiments but has lower hardness than Dwyer and others’ (1992) medium hardness solution without trace elements that was lethal to striped bass.
Mercury was less than the reporting limit of 0.0003 mg/L in all of the waters tested by Finger and others (1993). “In addition, aluminum, antimony, beryllium, bismuth, chromium, cobalt, copper, iron, nickel, silver, tin, titanium, thallium, and tungsten did not exceed reporting limits” (Finger and others, 1993, p. 25)..
A note on pH-adj: pH is defined as the negative logarithm, base 10, of the hydrogen ion concentration or, equivalently, reported as the negative of the exponent of the hydrogen ion concentration. Small changes in reported pH are very large changes in hydrogen ion concentrations. If pH in the toxicity profiles were normalized using standard units, pH 9 normalized to pH 8 would plot at a barely discernible 1.125 versus 1.0 when in fact the hydrogen ion concentration changed by a factor of 10. Consequently, pH-adj is 10 raised to the exponent of the reported pH value. pH-adj decreases with acidity as pH does.
Toxicity of Newlands Drain Water – top
As an indication of whether “Carson Lake and Pasture” waters are toxic, the graph of Fathead Minnow Toxicity Profiles for Stillwater National Wildlife Refuge, August 1987, and “Carson Lake” and Drains, shows “Sprig Pond” and “Carson Lake” Drain concentration profiles with those of 50% Hunter Drain, 50% “Lead Lake” (at 1.0), and D-Line Canal. The “Carson Lake and Pasture” waters generally plot between the most toxic and the least toxic samples of Finger and others (1993). If TDS, boron, molybdenum, or selenium concentrations are causes of mortality, then “Sprig Pond” and “Carson Lake” Drain are also likely toxic. High dissolved oxygen might be beneficial but high alkalinity probably isn’t based on the enrichments of 50% Hunter Drain and 50% “Lead Lake” water.
Data from Finger and others (1993); “Sprig Pond” and “Carson Lake” Drain analyses from Rowe and others (1991).
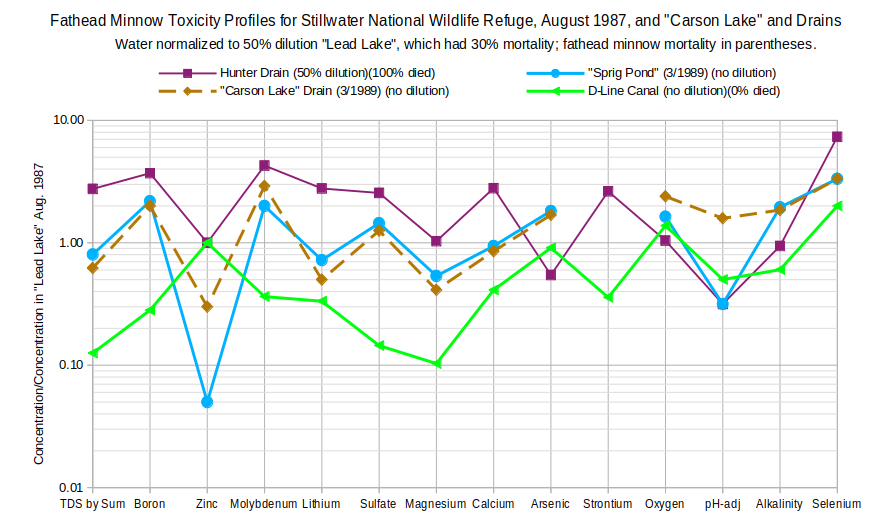
Trying to determine which solutes are the most important causes of death in the experiments of Finger and Others (1993) is complicated by the strong correlations of most potentially harmful constituents with TDS and, hence, with each other. Hunter Drain had the highest median concentrations of boron, lithium, molybdenum, and selenium but not arsenic. Hunter Drain also had the highest concentrations of sodium and potassium and the second highest concentrations of chloride and sulfate. Potassium has the highest positive correlation (n=6) with fathead minnow death rate in waters diluted to 50% but lithium, boron, selenium, sodium, and molybdenum are close behind (in descending order) with correlations greater than 0.89. For 4 degrees of freedom (n-2=4), a correlation coefficient greater than 0.88 is significant at the 0.01 level (1-tailed, on the assumption that mortality is caused by higher, not lower, concentrations). The problem is evaporative enrichment increases concentrations of all solutes, notwithstanding that some may be decreased to varying degrees by mineral-water reactions. As long as 1 death-causing solute increases with evaporation, many others go along for the correlation ride.
Toxicity of Newlands Drain Water – top
The toxicity of drain water upstream of “Carson Lake and Pasture” was confirmed in 10-day tests by Higgins and Miesner (2002) using Daphnia magna a few years later. To reveal seasonal toxicity changes, they collected samples from drains in March, May, and August. The March samples show results similar to those of Finger and others (1993). The L Drain at Pasture Road water used for normalization had a death rate of 50%. Waters with similar (i.e., 60%) or higher mortality rates had higher concentrations of TDS, boron, copper, molybdenum, and lithium (see graph of D. Magna Toxicity Profiles of March 1995 Drain Waters Near Stillwater or “Carson Lake” – Higher Mortality Results). All but Stillwater Diversion Canal had higher uranium. Low pH and oxygen are also associated with higher mortality. Results for arsenic and alkalinity are mixed.
Data from Higgins and Miesner (2002); “Sprig Pond” analysis from Rowe and others (1991).
Drains in and upstream of Stillwater National Wildlife Refuge: S2G Drain at Stuart Road near “Harmon Lake”, “Kent Lake” Drain at Freeman Road near Stillwater, “Stillwater Point Reservoir” Diversion Canal, Harmon Drain at Ditch House Road.
Drains and lakes in and upstream of “Carson Lake and Pasture”: “Carson Lake” 1 Drain on Pasture Road, L Drain at Pasture Road and Depp Lane, “Sprig Pond”.
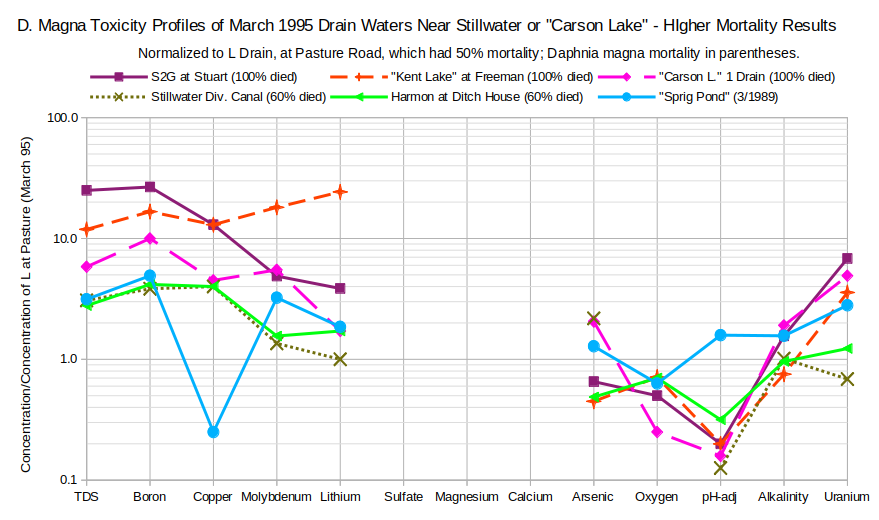
Higgins and Miesner (2002) did not analyze for calcium, magnesium, or other major ions except bicarbonate. The effects of hardness are unknown and evidently weren’t of interest.
Higgins and Miesner (2002) determined alkalinity in addition to bicarbonate. For the 34 samples I used for analysis, the average percent difference was -16% (alkalinity minus bicarbonate over the average of the 2). 68% of the differences were between -15 and -20%. Because alkalinity is a measure of bicarbonate concentration as well as concentrations of other bases, it should be greater than the concentration of bicarbonate (see note on alkalinity above). Only 2 of the 34 samples had alkalinity greater than bicarbonate. Although alkalinity less than bicarbonate concentration might indicate an analytical problem, the 0.98 correlation coefficient and small average difference indicates the 2 can be interchanged without compromising the interpretation.
The March “Sprig Pond” concentration profile hews closely to the drain samples with 60% mortality for TDS, boron, and lithium. It has much higher pH, higher molybdenum and uranium, higher or lower arsenic, and much lower copper (below detection limit). “Sprig Pond’s” alkalinity enrichment is closer to the more toxic waters while oxygen enrichments are close to both more and less toxic waters.
The concentration profiles for 6 more March 1995 drain waters with lower mortality show less consistent results than the higher mortality waters. On the graph of D. Magna Toxicity Profiles of March 1995 Drain Waters Near Stillwater or “Carson Lake” – Lower Mortality Results, TDS, boron, copper, molybdenum, and uranium enrichments in waters with lower mortality may plot above those of L Drain (e.g., Lower Diagonal at Pasture Road) and enrichments in waters with similar mortality may plot well below those of L Drain (e.g., Upper West Side Drain). Although “Carson Lake” Drain had only 33% mortality, its profile is close to that of L Drain, which had 50% mortality. For no solute are the concentration rankings the same as the mortality rankings for these waters.
Data from Higgins and Miesner (2002).
Drains in and upstream of Stillwater National Wildlife Refuge: Harmon Drain at highway NV 116.
Drains upstream of “Carson Lake and Pasture”: Upper West Side Drain at Solias Road, Sheckler Drain at St. Claire Road, “Carson Lake” Drain above “Carson Lake”, Lower Diagonal Drain at Pasture Road and Depp Lane, and L Drain above Lee Drain.
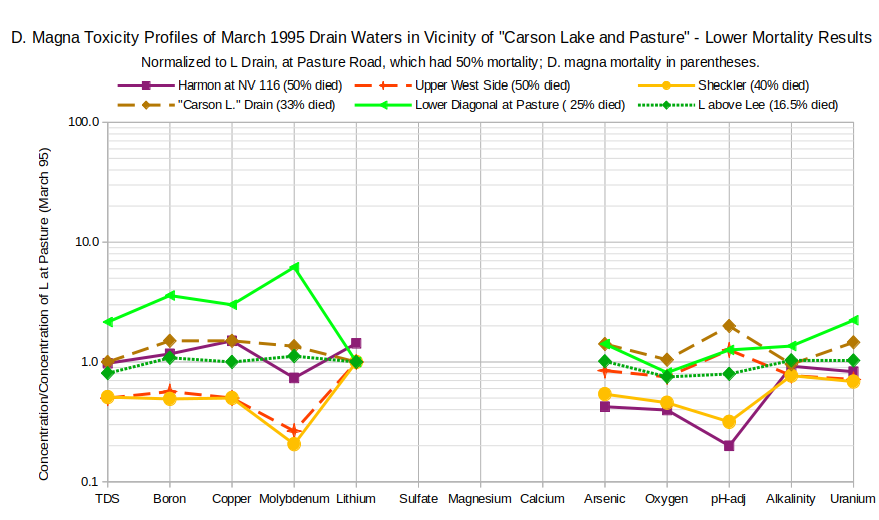
As in the data of Finger and others (1993), there are numerous strong correlations between solute concentrations in March samples and death rates of D. magna. Positive correlations of boron, chromium, copper, uranium, TDS, and bicarbonate with mortality (in descending order) are significant at the 0.01 level (1-tail) with correlations of 0.584 to 0.740 (n=15, 13 degrees of freedom). Not coincidentally, boron, chromium, copper, and uranium have the strongest correlations with TDS (greater than 0.775) at significance levels that are off the charts (mine only goes to 0.0005).
Toxicity of Newlands Drain Water – top
Other solutes have lower but still significant correlations with mortality. Nickel (0.557) and molybdenum (0.545) correlations with mortality are significant at the 0.025 (0.5139) level and zinc (0.474) and lithium (0.460) follow up at the 0.05 (0.4409) level of significance. Significantly (in the other sense), arsenic (0.148) has a very weak correlation and the aluminum correlation is even weaker and negative. The strong negative correlation of pH (-0.544) with D. magna death rates may point to environmental controls of trace element effects on aquatic animals. For example, at constant hardness and dissolved organic carbon, copper becomes more toxic as water becomes more acid (Appendix G in U.S. Environmental Protection Agency, 2007, Aquatic Life Ambient Freshwater Quality Criteria – Copper).
Correlations may have been unduly influenced by 2 of the March samples. They had TDS concentrations of 10,600 and 22,300 mg/L and hold the maximum concentrations of barium, boron, chromium, copper, lithium, molybdenum, nickel, uranium, and zinc. Both also had 100% death rates. The next highest TDS in the March group of samples was 5,200 mg/L. I deleted both of the highest TDS samples to see how the correlations would change. The correlation of TDS with mortality decreased but was still significant at the 0.05 level. Boron and uranium correlations with mortality slid to the 0.01 and 0.025 levels of significance, respectively, and chromium to the 0.05 level. Copper, molybdenum, and nickel fell to insignificance. Lithium and zinc correlations turned slightly negative. While the deadly consequences of the highest TDS waters shouldn’t be ignored, finding the real causes among many correlated potentially harmful trace elements is a challenge.
The “good” story of death by boron, chromium, uranium, and other solutes proffered by the March samples starts to fall apart in May. May drain waters which had higher death rates of D. magna generally tended to have higher concentrations of TDS, boron, copper, molybdenum, lithium, and uranium but concentration rankings fail to match mortality rankings for any solute. For example, 100% lethal S2G drain water had the highest boron concentration but “Carson Lake” 1 Drain, with 33% mortality, had second highest and Harmon Drain at Ditch House Road, with 65% mortality, comes in third. Waters with 80%, 60%, and 45% death rates are clustered below these. Correlations with mortality were weaker than in March even as correlations of trace elements with TDS were stronger and the correlation of TDS with mortality was good at the 0.01 level of significance. Copper (0.633), nickel (0.629), and zinc (0.632) supplanted boron and uranium with the highest positive correlations with death rates (n=12, 10 degrees of freedom, 0.01 level of significance). Boron, chromium, and uranium correlations are still significant, but less so, and the lithium correlation has risen to join them. Molybdenum’s correlation fell to insignificance.
The script flipped completely with the August samples. After about 4 months of releases from “Lahontan Reservoir”, the water coming down the drains had improved dramatically. Median TDS concentrations dropped from 2,370 mg/L (n=15, average 3,901 mg/L) in March to 499 mg/L (n=13, average 853 mg/L) in August and other solute concentrations fell accordingly. That should have been good for D. magna but it wasn’t. The 68% average death rate for Higgins and Miesner’s (2002) August samples is even greater than the 54% average death rate for the March samples. Correlations of TDS, boron, chromium, copper, uranium, zinc, nickel, molybdenum, and lithium with death rates were all negative and the negative nickel correlation is even significant at the 0.05 level. The remarkable changes weren’t due to pesticides. Higgins and Miesner (2002, p. 20) tested for pesticides in August and found that “concentrations of pesticides detected in drain-water were never near levels associated with aquatic toxicity”.
Toxicity of Newlands Drain Water – top
It is possible that most of the trace element concentrations in the drain and lake waters of “Carson Lake and Pasture” and Stillwater National Wildlife Refuge are too low to affect mortality of Daphnia magna and fathead minnows and all the apparent correlations with death rates are just noise. The table of Trace Element Concentrations in Drain and Lake Waters Near “Carson Lake and Pasture” and Stillwater National Wildlife Refuge and Some Water Quality Criteria compares the medians and ranges of trace element concentrations in waters draining to Stillwater Marsh and “Carson Lake” with various water quality criteria.
Stillwater drain sample sites: Lower Diagonal Drain at US 50, “Stillwater Point Reservoir” Diversion Canal, S2G Drain at Stuart Rd, Harmon Drain at NV 116, Harmon Drain at Ditch House Rd, S5A Drain at Austin Rd, Paiute Diversion Drain near Reservation, Stillwater Slough at Stillwater, “Kent Lake” Drain at Freeman Rd
“Carson Lake and Pasture” drain sample sites: Upper West Side Drain at St. Claire Rd, South Branch Carson River at St. Claire Rd, L Drain above Diagonal Drain, L Drain above Lee Drain, Lower Diagonal Drain at Pasture Rd, “Carson Lake” Drain above “Carson Lake”, Holmes Drain, Rice Drain, G-Line Extension Drain
“Carson Lake” sample sites: “Sprig Pond”, Islands Unit, Big Water Unit
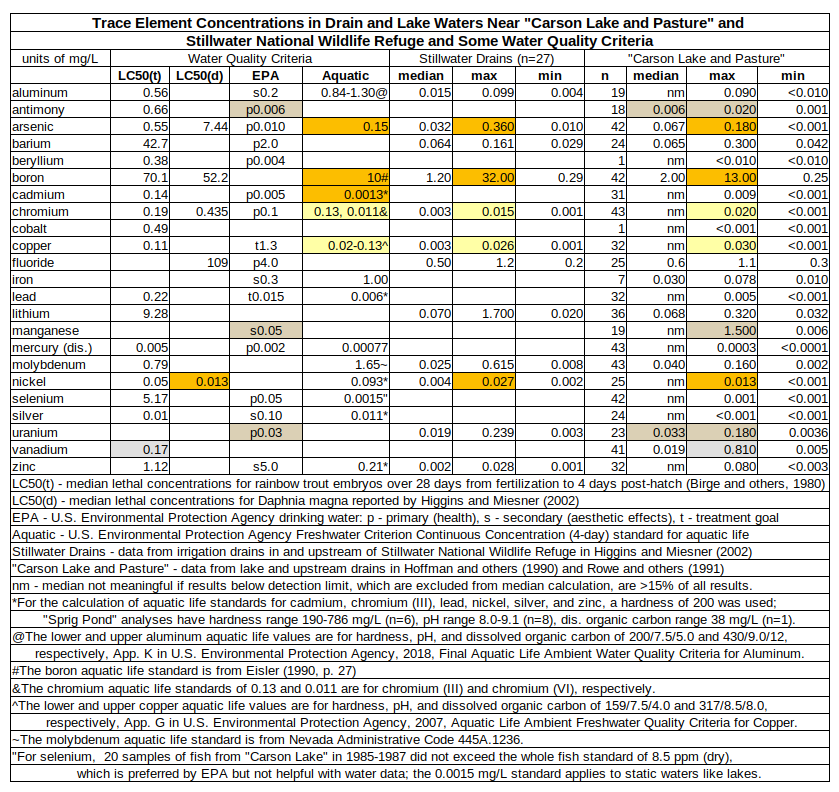
- (white) Observed drain concentrations are less than any available standards for aluminum, barium, beryllium, cobalt, fluoride, iron, lead, lithium, mercury, molybdenum, selenium, silver, and zinc
- (gray) The 0.810 mg/L maximum concentration of vanadium is unreliable. No other sample had a concentration greater than 0.060 mg/L.
- (tan) Observed drain concentrations are less than aquatic life standards and median lethal concentrations but greater than drinking water standards for antimony, manganese, and uranium.
- The manganese standard is for aesthetic effects and not relevant to aquatic animal mortality.
- Antimony and uranium have primary drinking water standards due to health effects but they are not an issue for aquatic animals until someone shows that they are.
- yellow) Maximum, but not median, observed drain concentrations are greater than parts of aquatic life standards for chromium and copper but those parts may not be controlling.
- Maximum concentrations in Stillwater and “Carson Lake and Pasture” drains exceeded the aquatic life standard for chromium (VI) but not for chromium (III). Chromium (VI) is less common in natural environments than chromium (III) (Higgins and Miesner, 1995, p. 15) and is probably significantly less than the total concentrations reported.
- Maximum copper concentrations in Stillwater and “Carson Lake and Pasture” drains exceeded the aquatic life standard calculated with the lowest likely hardness, pH, and dissolved organic carbon concentrations but not the standard calculated with higher parameter values. Copper is less dangerous at higher hardness, higher pH, and higher dissolved organic carbon. The close proximity of the maximum concentrations to the lower bound of the aquatic life standard suggests the probability of deaths to copper is low.
- (orange) Maximum, but not median, observed drain concentrations are above an aquatic life standard for arsenic, boron, cadmium, and nickel.
- 3 of 46 arsenic concentrations in Stillwater drain waters and 1 of 47 in “Carson Lake and Pasture” drain waters exceeded the aquatic life standard for arsenic of 0.15 mg/L. 2 other Stillwater analyses and 6 other “Carson Lake” analyses had arsenic concentrations greater than 0.10 mg/L.
- 4 of 46 boron concentrations in Stillwater drain waters and 2 of 47 in “Carson Lake and Pasture” drain waters exceeded an aquatic life criterion for boron of 10 mg/L. 3 other Stillwater analyses and 1 other “Carson Lake” analyses had boron concentrations greater than or equal to 7.5 mg/L. Eisler’s (1990) criterion for boron may not be well established. National agencies have apparently not established aquatic life standards for boron.
- 2 of 31 cadmium concentrations in “Carson Lake and Pasture” drain waters exceeded the aquatic life standard but 27 results were below the detection limits. The only measured concentrations were 0.001, 0.001, 0.008, and 0.009 mg/L.
- Maximum nickel concentrations in Stillwater and “Carson Lake and Pasture” drains exceeded the median lethal concentration of 0.013 mg/L reported by Higgins and Miesner (2002, p. 17) for Ceriodaphnia dubia. Higgins and Miesner (2002, p. 17) also cited a study which found median lethal concentrations of about 0.003 mg/L for Daphnia magna at a hardness of 117 mg/L. Standards based on single experiments are less reliable than aquatic life standards.
The observations above must be qualified by the detection/reporting limits of the analyses. Aquatic life standards for cadmium, chromium, and selenium were less than 2 times the detection limits and that for copper was exactly 2 times the detection limit.
Although the data of Trace Element Concentrations in Drain and Lake Waters Near “Carson Lake and Pasture” and Stillwater National Wildlife Refuge and Some Water Quality Criteria do not indicate any single trace element is a likely cause of fish or cladoceran deaths, they do not address the possibility that certain combinations of trace elements are. Without large numbers of controlled experiments with various combinations of trace elements, determining the cause(s) of death in the toxicity experiments will remain elusive.
Probably the best case that can be made for a harmful trace element in Newlands drain water is the one for arsenic. At least a few drain or lake water analyses have exceeded the aquatic life standard and median concentrations exceed the primary drinking water standard. Nonetheless, the toxicity profiles show that waters causing higher mortality often have arsenic concentrations lower than those causing lower mortality. In the data of Higgins and Miesner (1993), arsenic did not have a significant correlation with death rates for Daphnia magna.
The experiments of Dwyer and others (1992), Finger and others (1992), and Higgins Miesner (2002) have demonstrated that Newlands drain waters and the receiving lakes of Stillwater National Wildlife Refuge and “Carson Lake and Pasture” are toxic to some species of fish and cladocerans to varying degrees. The waters have relatively high concentrations of several potentially harmful trace elements. Some major ion concentrations may also be harmful. No individual solutes have been identified as causes of death. It may be that the interactions of several solutes account for the toxicity but the deadliest combinations have not been determined. In any case, higher mortality is associated with higher TDS and higher TDS is caused by evaporation, which affects all solutes.
Toxicity of Newlands Drain Water – top