Sediments which underlie Carson Desert are an obvious potential source of constituents in the water of “Carson Lake and Pasture”. The basin of the Carson Sink is filled with sediments and minor volcanic rocks to a depth greater than 2,400 m. An oil test well was drilled to 8,001 feet without hitting bedrock (Glancy and Katzer, 1976, p. 14). Only the uppermost, generally unconsolidated sediments are relevant to surface water quality. Morrison (1964, p. 18) identified 2 major stratigraphic units in the uppermost 100 m (330′) of the basin fill: the Fallon formation and, below it, the Lahontan Valley group. These are informal geologic names. Bell, Caskey, and House (2010) referred to these units as the Fallon Alloformation and the Lake Lahontan sequence.
Lake Lahontan was the coalescence of Pyramid Lake, Winnemucca Lake, Honey Lake, Walker Lake, and lakes periodically filling the basins of Smoke Creek Desert – Black Rock Desert, Desert Valley, Quinn River Valley, Buena Vista Valley, and the Carson Desert (Benson, 1994, Figure 2). It was the result of climate change during and following the last major glacial advance, the Wisconsin, beginning about 35,000 years ago (Benson, 1994, Figure 2, p. 2, and Figure 3, p. 3). The lake was up to about 150 m (490′) deep in the Carson Sink at its deepest about 15,000 years ago (1,330 m water level on Benson’s Figure 3 to Carson Desert floor of 1,180 m). Water levels fluctuated over the millennia. Parts of the lake were dry at times. The water level of Lake Lahontan dropped rapidly 13,000-14,000 years ago and since that time lakes in Carson Sink have been separated from Pyramid Lake (Benson, 1994, Figure 3, p. 3).
The Lahontan Valley group consists mostly of fine sand, silt, and clay, local beach gravel, and minor river-channel and river-delta sand deposited in Lake Lahontan. Thin wind-blown sand was deposited at the top of the group after Lake Lahontan had dried up (Morrison, 1964, p. 75). In the Carson Desert, Morrison (1964, p. 28) inferred 5 deep-lake to shallow- or no-lake cycles during deposition of the Lahontan Valley group. Morrison (1964, p. 9) measured a maximum thickness of 100 m (330′) for the group but that doesn’t mean that much is present at every location or that the group isn’t thicker in some locations.
The sediments of the Lahontan Valley group are derived from the mountains surrounding the Carson Desert. Alluvial fans of mostly coarse material accumulated at the feet of the mountains above the shore lines of Lake Lahontan. Debris flows no doubt entered the lake at times. Most of the sediments washed into the lake came from the Carson River. The chemical character of the Lahontan Valley group is consequently dominated by the rocks exposed in the Carson River drainage.
The Fallon formation includes sand, silt, and clay deposited in shallow, relatively short-lived lakes; sand, silt, and gravel deposited by various Carson River channels and deltas; and wind-blown sand (Morrison, 1964, p. 28). The sediments of this formation provide evidence of 5 shallow lake periods in the Carson Sink and intervening periods of dryness and a following period of complete dryness (Morrison, 1964, p. 80-81). Morrison (1964, p. 9) reported a maximum thickness of 11 m (35′) for the Fallon formation but the presence or absence of various river deposits, sand dunes, and wind-deflation hollows cause major changes in thickness from place to place.
Dating of organic matter in the Fallon formation by analyzing the concentrations of the radioactive isotope of carbon, carbon-14, indicate that a lake was present in the Carson Sink during the periods: 1,500-1,900 years ago, 2,000-3,900 years ago, 3,900-4,900 years ago, and 4,900-6,700 years ago (Bell and others, 2010, p. 12). A buried ash bed indicates the oldest lake formed more recently than the explosion of Mount Mazama about 6,900 years ago. That explosion formed Crater Lake, which is now a national park in Oregon.
Sediments of Lake Lahontan – top
Some of the characteristics of the sediments of the Lahontan Valley group and Fallon formation that are particularly relevant to water quality are the presence of the easily soluble minerals calcite and gypsum and the presence of organic-rich silt and clay. When calcite dissolves, it releases ions of calcium and bicarbonate (HCO3) or carbonate (CO3). Gypsum releases calcium and sulfate (SO4) ions. Both calcite and gypsum precipitate when water has evaporated sufficiently but Morrison (1964) did not describe distinct layers of these minerals. Instead, calcite is common and widely distributed in the sediments as tufa coatings and crusts on rocks in beach gravels and on former lake bottoms, as caliche-like layers in current and former soil horizons, as nodules, as intergranular disseminations, and as shells of ostracods, gastropods (i.e., snails), and pelecypods (i.e., clams) (see descriptions of stratigraphic sections, p. 121-143). Gypsum is less common but was noted in stratigraphic section descriptions on pages 128, 135, 129, 131, 133, and 143, at least, of Morrison (1964). It apparently occurs primarily as intergranular disseminations and segregations.
Halite, or table salt, is precipitated from evaporating water after calcite and gypsum and is very soluble. It releases sodium and chloride ions when dissolved. Morrison did not identify it in the Lahontan Valley group or Fallon formation. However, Morrison (1964, p. 133) did write that a silt layer with abundant gypsum in the Fallon formation had a “slightly salty taste”. He used the term “saline” to refer to unspecified minerals on several occasions (e.g., pages 121, 128, 131, 140). This could mean he couldn’t identify the mineral but its crystal form suggested it precipitated from water. These saline minerals could include, or be in addition to gypsum.
A test well found a 2 m (7′) thick layer of halite at the surface of the Sand Springs deposit in the Salt Wells Basin southeast of Carson Sink (U.S. Geological Survey and Nevada Bureau of Mines and Geology, 1964, p. 250). This layer has apparently never been mined. Nearby, halite has been produced by evaporation of water accumulated during the winter and spring run-off and is still being produced by Huck Salt Company. In 2011, Huck Salt Company produced 21,000,000 kg (“23,618 tons”) of sodium chloride (Visher and Coyner, 2012, p. 17).
Borax, which contains boron, is another very soluble mineral precipitated from evaporating water that was also found in the Sand Springs area. About 900 kg (1 ton) were produced in 1870-1872 (U.S. Geological Survey and Nevada Bureau of Mines and Geology, 1964, p. 184). The floor of Salt Wells Basin is slightly higher than that of Carson Sink but it was inundated by Lake Lahontan during the same periods of time. It is thus possible that layers of halite and borax are present in the shallow sediments elsewhere in the Carson Desert.
Organic matter in the Lahontan Valley group and the Fallon Formation has likely accumulated, by adsorption, significant concentrations of trace elements that don’t fit well into common minerals. Lake evaporation at various times no doubt facilitated that process. The adsorbed trace elements can later be desorbed, such as when the organic matter is oxidized by contact with surface water. Black, carbonaceous matter is noted by Morrison (1964) in several of the stratigraphic sections he described (e.g., p. 130, 133, 134, 139), principally associated with fine sand, silt, or clay deposited on a lake bottom. The most organic-rich sediments have “a strong peaty, and in places, hydrogen sulfide odor” (Morrison, 1964, p. 37). In well logs, they are referred to as “black gumbo”, “black tule sand”, and similar terms. Well log 38L (p. 149) has 31 m (101′) of “clay, black”. Well log 51L (p. 149) has 24 m (79′) of “gumbo, black” including 4.6 m (15′) with “gas showing”. Bell and others (2010, p. 12) also noted “organic-rich muds, or ‘black mats'” but interpreted some as being deposited by Carson River flood waters on the flats outside the river channels rather than on the bottom of a lake.
Sediments of Lake Lahontan – top
With that background, one can compare the abundances of trace elements in Lake Lahontan sediments to global averages for various rock types to see if Lake Lahontan sediments have unusually high concentrations of certain elements. The trace elements relevant to ecosystem health are arsenic, boron, lithium, mercury, molybdenum, selenium, and uranium (Tuttle and others, 2020, p. 2). The table of Abundances of Trace Elements of Concern in Sediments of the Carson Desert shows that the median concentrations of arsenic, lithium, and uranium in Carson Desert sediments are similar to concentrations in global average shale. Low selenium and molybdenum concentrations may be due to a higher proportion of granitic or intermediate igneous rocks, or both in the Carson River drainage area than in the drainage areas of the shales used to compute the global average. Hoffman and others (1990, p. 7) noted that soils in the western United States have arsenic and selenium concentrations commonly in the range 1.2-22 ppm and 0.039-1.4 ppm, respectively. Concentrations in Carson Desert sediments fall within these ranges. The median mercury concentration of Carson Desert sediments does not reflect the anthropogenic contamination and may also be lower than global average shale due to the particular mix of rock types in the Carson River drainage.
Carson Desert data are from Tidball and others, 1991, U.S. Geological Survey, Open-File Report 91-584A.
“Carson Lake” data are from Hoffman and others, 1990, U.S. Geological Survey, Water-Resources Investigations Report 89-4105.
Data on global averages for various rock types are from Parker, 1967, U.S. Geological Survey, Professional Paper 440-D.
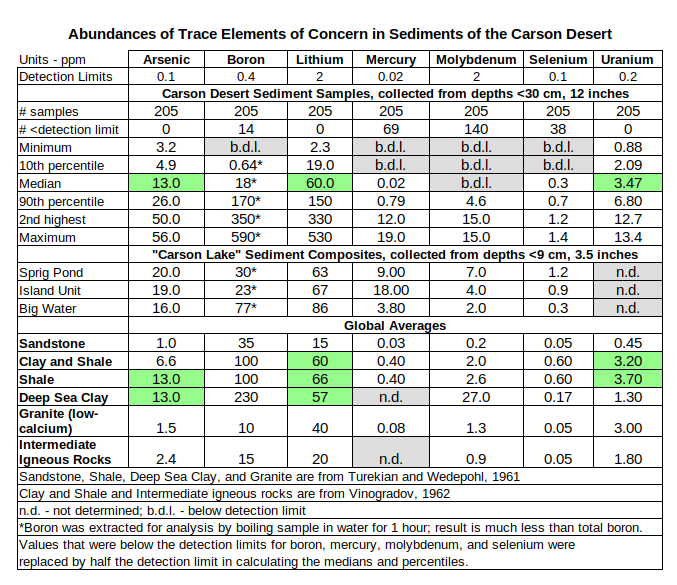
The median boron concentration is less than that in shale due to different analytical methods. Hot-water extractable boron was measured for Carson Desert sediments and total boron was measured for global average shale. Stewart and others (1989) showed that hot-water extractable boron was 2-47% of total boron in 69 soil samples from the western United States. In particular, the geometric mean of hot-water extractable boron for 2 samples from the Stillwater Wildlife Management Area was 1.5 ppm and the geometric mean of total boron was 20 ppm. Boron analyses for agricultural purposes overwhelmingly use hot-water extraction or comparable partial methods.
Sediments of Lake Lahontan – top
The maximum values for boron and lithium may be outliers as they are more than 60% higher than the second highest values. However, the boron and lithium maximums are from the same sample and the distributions of boron and lithium have very long tails. 12 samples had boron concentrations greater than 200 ppm and 18 had lithium concentrations greater than 150 ppm. It may be that there is a distinct sediment type with very high relative boron and lithium concentrations but that it is rare in the survey area. Arsenic and uranium also have asymmetric distributions with long tails as well but their distributions are not as extreme as those of boron and lithium.
Sample sites for Carson Desert sediments were selected randomly (Tidball and others, 1991, p. 1 and Figure 1, p. 8) in order to satisfy certain assumptions for statistical analysis. Consequently, areal coverage is not uniform and the medians and percentiles may not apply to smaller geographic areas within Tidball and others’ (1991) survey area. In fact, trace element concentrations for all 3 locations for “Carson Lake” sediments in Hoffman and others (1990) are higher than the medians for Tidball and others’ (1991) samples. All 3 mercury concentrations, 1 molybdenum concentration, and 2 selenium concentrations from “Carson Lake” are higher than the Carson Desert’s 90th percentiles.
Fallon formation and Lahontan Valley group sediments in the Carson Desert have median trace element concentrations that are not unusually high. Most of the medians are close to global averages for shale. Both were deposited as mostly silt and clay at the bottom of water bodies, either lakes or oceans. Generally low concentrations of mercury, molybdenum, and selenium compared to global average shale may indicate that the Carson River drainage has a greater proportion of granitic and intermediate igneous rocks than the source areas of global average shale. The long-tailed distributions of Tidball and others’ (1991) results and other data suggest that there are hot spots for certain elements within the Carson Desert, particularly mercury. In any case, the sediments are a warehouse of major, minor, and trace elements available for dissolution. Depending on water chemistry, one or more of these elements could be leached into ground water up to potentially harmful concentrations.
Sediments of Lake Lahontan – top