What’s in the Names Fairy Shrimp and Anostraca?
Branchiopods Are Crustaceans Are Arthropods
Taxonomy within Branchiopoda
What Distinguishes the Order Anostraca from the Other Branchiopod Orders?
Do the Branchiopod Orders Have Different Habitats?
Do the Branchiopod Orders Use Different Feeding Behaviors?
When Did Crustaceans and Other Arthropod Subphyla First Appear on Earth?
When Did Branchiopods and Other Crustacean Classes First Appear on Earth?
When Did Anostracans and Other Branchiopod Orders First Appear on Earth?
How Did Early Anostracans Live?
The Great Game: Extinction and Survival
References are on a separate page: References.
This is not an up-to-date or comprehensive discussion of the taxonomy and fossil history of Anostraca and related taxonomic groups. It is inspired by R.C. Moore’s (editor) Treatise on invertebrate paleontology, Part R, Arthropoda 4, Vol. I (Geological Society of America, Boulder, 1969). I was surprised that so much was known about anostracans and branchiopods in general. Although imperfect and, at times, hard to understand, the fossil record is an amazing testimony to the profuse variation of life. Moore’s work is out of date now. I include here some of the publicly available updates I have come across but I don’t have access to most of the research.
What’s in the Names Fairy Shrimp and Anostraca?
Fairy shrimp are individuals of the order Anostraca, which translates to without a shell in English. The order Anostraca belongs to the class Branchiopoda. The English translation of branchiopod is lung feet. Due to the feathery or leaf-like legs, some branchiopods have also been called phyllopods, which means leaf feet.
Fairy shrimp and shrimp are both members of the subphylum Crustacea but they are otherwise not related. Shrimp are members of the class Malacostraca, subclass Eumalacostraca, Superorder Eucarida, and order Decapoda, which also includes crabs, lobsters, and crayfish. Members of the Infraorder Caridea may be referred to as true shrimp (en.wikipedia.org/wiki/Caridea) and includes species commonly eaten by humans. Species of the Infraorder Dendrobranchiata, particularly the family Penaeidae, are also eaten by humans and are commonly referred to as prawns. In North American culture, menu items of Caridea and Dendrobranchiata species are both commonly referred to as shrimp whereas in British and Australian cultures, they are both commonly referred to as prawns (en.wikipedia.org/wiki/Dendrobranchiata). Just as sea lions are not lions and koala bears are not bears, fairy shrimp are not shrimp, or prawns.
I have not read an etymological explanation of the “fairy” part of “fairy shrimp”. Without delving into the mythology of fairies, a quick guess would be that fairy shrimp are called “fairy” because they are small and may be hard to find in the British Isles, where I presume the term originated. They might have magical powers but I don’t know how I would test for that.
When a friend asked me about the difference between fairy shrimp and brine shrimp, I realized that question could be a source of confusion if I didn’t specifically address it. The parenthetical pairing of “fairy shrimps” with “Anostraca” by Belk (1975), Pennak (1978), and Thorp and Covich (2001) make it clear that all anostracan species are fairy shrimp. However, it is common practice for journal articles about species of the genus Artemia to refer to the animals as brine shrimp rather than as fairy shrimp. The same is probably true of the genus Parartemia but I have seen very few Parartemia articles. Timms (2012) went so far as to write, “Technically shrimps of the genera Artemia and Parartemia are termed brine shrimps”. Commercially, Artemia species are uniformly referred to as brine shrimp (Van Stappen, 1996). Artemia is the only genus of the family Artemiidae and Parartemia is the only genus of the family Parartemiidae. The Suborder Artemiina (Rogers, 2013) contains only these 2 families. Consequently, one could also say that all Artemiinids (Artemiinans?) are brine shrimp.
What is the salinity of the water that Artemia and Parartemia live in? The TDS ranges given by Eng, Belk, and Eriksen (1990) for Artemia franciscana and Artemia monica in California are 35,000-172,000 mg/L and 75,000-95,000 mg/L, respectively. Kaestner (1970, p. 90) gave an upper limit of 230,000 mg/L for Artemia. Timms (2012) summarized the TDS ranges for 15 Parartemia species in Australia. The highest concentration was 353,000 mg/L and the maximum observed was greater than 200,000 mg/L for 7 other species. The minimum observed concentrations were less than or equal to 35,000 mg/L for 12 of the 15 species but these likely occurred early in the lives of the host ephemeral ponds.
Species other than Artemia and Parartemia have been observed in waters with high TDS concentrations. Branchinecta campestris and Streptocephalus texanus have been observed in waters with 11,100 mg/L and 16,500 mg/L TDS, respectively (Total Dissolved Solids section on the Habitats of Fairy Shrimp page). In addition, Branchinecta campestris has been observed with Artemia by Lynch (1960), many others, and even by me in Monitor Playa Lake in Monitor Valley and in Screeching Avocets Pond in the Great Divide Basin. To my knowledge, B. campestris has not been referred to as brine shrimp.
What is brine? An article on “Brackish and Saline Groundwater in New Mexico” in the summer 2015 issue of New Mexico Earth Matters by the New Mexico Bureau of Geology (available online) defines brine as 35,000-200,000 mg/L. Most seawater has a TDS concentration of about 35,000 mg/L. The Wikipedia water salinity table (en.wikipedia.org/wiki/Salinity#SalinityTable) defines brine as having a concentration greater than 50,000 mg/L.
In summary, all anostracan species are fairy shrimp but those which are, at least at times, found in waters with TDS concentrations greater than 35,000 mg/L can be referred to as brine shrimp. On this web site, I follow Kaestner (1970, p. 90), Pennak (1978, p. 337), and Thorp and Covich (2001, p. 895) in using the genus name to refer to Artemia rather than the term brine shrimp. If the name Artemia is derived from the Greek goddess Artemis, who is associated with childbirth in addition to hunting and wild animals (e.g., www.theoi.com/Olympios/Artemis.html), I suspect it is because Artemia are prolific (see “Steamboat Lake” 2nd East Pond 1989-06-07, #0205) and not because of the salinity they thrive in.
Taxonomy and Origin of Anostraca – top
Branchiopods Are Crustaceans Are Arthropods
The subphylum Crustacea is within the phylum Arthropoda. See Anostraca Taxonomy Table 1 for a list of the taxonomic units considered here and the overall scheme of things; it is based on Moore (1969) but includes annotations for some more recent changes. The taxonomy (classification) of Crustacea and its classes has changed over the decades and is still changing. There are also still many questions about what goes where in its parent phylum, Arthropoda. To greatly oversimplify, the phylum Arthropoda is characterized by segmented bodies with paired appendages and the subphylum Crustacea by 2 pairs of antennae and respiration through gills or the body surface (Dexter, 1959, p. 558). Some have placed crustaceans along with insects and myriapods within the subphylum Mandibulata (Thorp and Covich, 2001, p. 12) due to their similar mouth parts with a pair of mandibles and a pair of maxillae (Dexter, 1959, p. 558).
The class Branchiopoda is distinguished from other crustaceans by mandibles lacking a palp and reduced maxillae for mouth parts, reduced or vestigial first antennae, and generally uniform, leaf-like appendages which function as gills (Kaestner, 1970, p. 84). Not that I know what palps and maxillae are. Members of this class also have paired eyes (except cladocerans), posterior trunk segments without appendages, and metamorphosis with a nauplius or metanauplius stage (Moore, 1969, p. R131). However, Olesen (2007) concluded that adult branchiopods have very few features that are common to all branchiopods but absent from all other crustaceans. He nonetheless proposed that branchiopods are a coherent taxonomic unit based on morphological details of larval antennae, larval and adult mandibles, and larval and adult legs (see list of synapomorphies on p. 170, Olesen, 2007).
Taxonomy and Origin of Anostraca – top
Taxonomy within Branchiopoda
There are several different kinds of branchiopods, not just fairy shrimp. Consequently, it is helpful to know the difference between fairy shrimp and other branchiopods and what the other branchiopods are named. There have been various ways to arrange the orders within the Branchiopoda class since fairy shrimp were described in the late 19th century. Pennak (1978, p. 327) grouped the orders Anostraca, Notostraca (tadpole shrimp), and Conchostraca (clam shrimp) into what he termed the Division Eubranchiopoda, which excluded Cladocera. Thorp and Covich (2001) seemed to support such a distinction. Indeed, S.I. Dodson and D.G. Frey, who wrote the chapter on branchiopods, titled the chapter “Cladocera and Other Branchiopods” . They also stated that the name cladocera no longer has taxonomic significance as the 4 orders of cladocerans are probably not closely related (Thorp and Covich, 2001, p. 850). The term cladocera is nonetheless helpful to collectively refer to members of the 4 orders, which differ from other branchiopods in having a carapace covering all but the head, a single eye, and only 4-6 pairs of thoracic appendages (Dexter, 1959, p. 559). Thorp and Covich (2001) added that cladocerans are not segmented (p. 851) and have a claw-bearing postabdomen, which is posterior to the anus (p. 855). In contrast, Preuss in the 1950s separated Anostraca from the taxonomic unit Phyllopoda, which included Notostraca, Conchostraca, and Cladocera (Olesen, 2007, p. 168). Others have more recently used this approach, including Walossek (1993) and Olesen (2007). Walossek (1993, p. 71) further distinguished the subclass Calmanostraca, with the orders Notostraca and Kazacharthra (now extinct), from the subclass Onychura, which included all the conchostracans and cladocerans.
Moore (1969) and Belk (1982) included Cladocera and the order Conchostraca in the subclass Diplostraca; the order Notostraca in the subclass Calmanostraca; and the order Anostraca in the subclass Sarsostraca. In addition, Moore (1969) placed the fossil orders Kazacharthra and Acercostraca (since reassigned to Phyllocarida, as reported by Walossek, 1993, p. 69) in Calmanostraca and fossil Lipostraca (genus Lepidocaris) in Sarsostraca. To support placing Cladocera in Diplostraca with Conchostraca, Moore (1969, p. 130) cited the similarity of adult cladocerans and some conchostracan larvae and the suggestion by others that Cladocera evolved as a neotenic form (sexual maturity attained in a larval stage) of a conchostracan ancestor.
Focusing on leg morphology and habitat, Olesen (2007) constructed a phylogenetic tree with Rehbachiella kinnekullensis fossils, which were discovered after Moore (1969), on a branch from the base of the branchiopod stem rather than within an extant subclass. Sarsostraca (including Anostraca and fossil Lepidocaris) branched off first and the stem became Phyllopoda. Calmanostraca (including Notostraca, fossil Castracollis, and fossil Kazacharthra) diverged next, leaving a Diplostraca stem. The clam shrimp orders Laevicaudata and Spinicaudata split off the Diplostraca stem, which then became Cladoceramorpha. Cladoceramorpha split into a branch for the clam shrimp order Cyclestherida and a terminal stem of Cladocera. Cladocera ultimately split into the orders Ctenopoda, Anomopoda, Onychopoda, and Leptodora (Thorp and Covich, 2001, p. 850, used the name Haplopoda instead of Leptodora).
Taxonomy and Origin of Anostraca – top
What Distinguishes the Order Anostraca from the Other Branchiopod Orders?
Anostracans (fairy shrimp) differ from other branchiopods in having rather cylindrical bodies (ignoring the legs) with no carapace, pairs of stalked rather than non-stalked eyes, and 11 pairs of thoracic appendages (except one family that has 17 or 19), or legs (Pennak, 1978, p. 327; Dexter, 1959, p. 559; Kaestner, 1970, p. 85). A distinctive feature of anostracans is the enlarged and sometimes intricate development of the second antennae of males, which are used to grasp females during copulation (Linder, 1941, p. 127-145; Belk, 1975). Unlike other branchiopods, fairy shrimp typically swim with backs down (i.e., legs above the body and toward the water surface; Pennak, 1978, p. 329-330). Most anostracans are 1.0-2.5 cm (0.4-1″) long but some are as small as 0.5 cm (0.2″) and the 3 predatory species are 5-10 cm (2.0-3.9″) long (Dexter, 1959, p. 560; Rogers and Timms, 2017). Fossils of the other Sarsostraca order, Lipostraca, are only about 0.3 cm (0.1″) long (Moore, 1969, p. R183).
Notostracans (tadpole shrimp) are morphologically very different from anostracans. In spite of the common name, they don’t look like tadpoles, they look like little horseshoe crabs. They have a flexible, shield-like carapace covering the head and most of the body. Their 2 eyes are close together in the middle of the front part of the carapace. The thorax and forward abdominal segments have pairs of legs and some have more than one pair. The rearward abdominal segments lack legs and are not covered by the carapace. The number of segments and pairs of legs (up to 71) varies, even within a species. The antennae are inconspicuous but the first (or first 2) pair(s) of legs look like antennae as they have long, whip-like branches that generally stick out laterally from under the carapace. There are 2 conspicuous, long, thin, segmented, tail-like features that extend from the end of the body to form an acute angle (Kaestner, 1970, p. 95-97; Pennak, 1978, p. 328-329). The bodies are 2-10 cm (0.8-3.9″) long (Belk, 1982). For more information, visit the Other Crustaceans You May Find With Fairy Shrimp and Tadpole Shrimp Videos pages.
Conchostracans (clam shrimp) are also very different morphologically from anostracans. Their bodies are flattened laterally within a hinged carapace. The 10-32 pairs of legs (Kaestner, 1970, p. 99) do not protrude from between the valves but pairs of bristly branches of modified second antennae do and they are used for swimming instead of the legs (Pennak, 1978, p. 329). The legs are primarily used for feeding and respiration. The end of a clam shrimp body has a pair of stout anal spines (Pennak, 1978, p. 328), which are still within the carapace. Conchostracan carapaces are up to 2 cm (0.8″) long (Belk, 1982). For more information, visit the Other Crustaceans You May Find With Fairy Shrimp and Clam Shrimp and Ostracod Videos pages.
The morphological features of cladocerans mentioned in the first paragraph under Taxonomy within Branchiopoda above are sufficient to distinguish them from anostracans. Most are less than 0.6 cm (0.2″) long. Some are less than 0.2 cm (0.08″). One predatory species is up to 1.8 cm (0.7″) long (Belk, 1982). For more information, visit the Other Crustaceans You May Find With Fairy Shrimp page.
Taxonomy and Origin of Anostraca – top
Do the Branchiopod Orders Have Different Habitats?
The branchiopod orders cannot be distinguished by habitat. Most species from each of the branchiopod orders inhabit ephemeral, non-flowing waters. These are typically small ponds that form from snowmelt in the spring or from high precipitation events at other times of year. Large ephemeral lakes with opaque, clay-rich water (e.g., playa lakes) also host multiple orders of branchiopods. Some cladocerans live in fast-moving streams (Thorp and Covich, 2001, p. 860) and a few species live in the sea (Belk, 1982). Anostracans, at least, inhabit perennial mountain lakes and a few species inhabit permanent lakes saltier than sea water (e.g., Great Salt Lake and Mono Lake) but I haven’t read of other branchiopods in such habitats. Species from different orders sometimes occur in the same pond at the same time.
Branchiopods generally do not co-exist with fish because they are easily seen and not fast swimmers. Exceptions are some cladocerans and some clam shrimp which have been reported in goldfish-rearing ponds in California by Pennak (1978, p. 337).
Taxonomy and Origin of Anostraca – top
Do the Branchiopod Orders Use Different Feeding Behaviors?
Feeding is a major control on animal morphology and, hence, taxonomy. The branchiopod orders have specialized in different feeding behaviors and families within certain orders also exhibit different feeding behaviors. This complicates the process of identifying characteristics common to all branchiopods and also in comparing branchiopods to other crustacean classes, many of which have also spread into different habitats using different feeding methods.
Anostracans are filter feeders which sweep small suspended phytoplankton and zooplankton from the water column for food. They have generalist legs which are used for both feeding and propulsion. There are 3 predatory anostracan species (Rogers and Hill, 2013; Rogers and Timms, 2017). They are apparently still filter feeders but they grow large enough to capture other anostracans and large zooplankton without much change in their swimming routine. Moore (1969) did not note any anatomical differences for the predators (only 1 species at that time); nor did Kaestner (1970), Pennak, (1978), or Belk (1982). Belk (1982) noted that some anostracans occasionally use their legs to scrape food from “surfaces” (i.e., pond bottoms) but did not note any phenotypic changes in leg morphology.
Notostracans are generally not filter feeders and their legs differ in type and number from those of anostracans. Notostracans are principally bottom feeders. They ingest organic matter and small organisms stirred up from the bottom mud by their legs but they also have sharp mandibles for eating other branchiopods, cladocerans, ostracods, insect larvae, and amphibian eggs they come across (Kaestner, 1970, p. 98-99). Kaestner’s (1970, p. 98) colorful description of their feeding behavior is: “they throw themselves over the prey, covering it with the whole body” and “rasp the prey with their sharp endopodites and distal endites” of the legs . The first 11 pairs of legs are larger and stiffer than the rest and are used for swimming, crawling, and climbing up vegetation (Kaestner, 1970, p. 96, 98). These legs are also used for feeding, as in anostracans (Kaestner, 1970, p. 98-99). Kaestner (1970) did not say what the other legs are used for. A bottom-dwelling life may make a shield-like carapace that would be cumbersome for swimming more advantageous as camouflage or protection from attacks from above.
Conchostracans are mostly bottom feeders but at least one genus is a filter feeder. A major difference from anostracans and notostracans is that a modified second antenna rather than the legs are used for swimming (except for the filter feeding genus) (Kaestner, 1970, p. 102). The legs are small and enclosed by the bivalve carapace (Kaestner, 1970, p. 99-101). They are still used for filtering small food particles from the water but this occurs between the valves, which are opened slightly to allow water to enter. The smaller legs toward the rear of the body seem to be used to push coarse particles out of the food stream (Kaestner, 1970, p. 102). Superficially, the shell, antennal swimming appendages, and a single eye rather than 2 indicate a conchostracan lifestyle different from those for anostracans and notostracans. Nonetheless, the basic branchiopod body plan has adapted to the occasion.
Cladocerans are a step farther from anostracans and notostracans and differ from conchostracans with smaller body sizes and carapaces that do not cover the entire body. Like conchostracans, they use their second antennae for swimming (Belk, 1982). They may have the same types of feeding behaviors but at a different scale and in a greater variety of habitats. Most cladoceran species are herbivorous but some are predatory. The herbivorous cladocerans generally eat bacteria, algae, protozoa, organic matter particles, and copepod nauplii up to 0.1 mm (0.004″) by filter feeding. Most ingested food particles are less than 0.025 mm (0.001″). Some climb up aquatic plants for filter feeding or scrape the plant surfaces. Some crawl along or into the bottom mud. The predatory cladocerans eat rotifers, other cladocerans, copepods, small larvae, and crustaceans up to 1.5 mm (0.06″). Their diets may also include algae and protozoa (Thorp and Covich, 2001, p. 866). Cladocerans inhabit a greater variety of habitats than other branchiopods, such as lakes with fish (Thorp and Covich, 2001, p. 866-867), streams, even groundwater (Thorp and Covich, 2001, p. 860), and the sea. Fish are visual size-selective predators (as reported in Dodson, 1970) so the cladocerans which co-exist with fish are smaller than those which don’t. This suggests the cladoceran variations on the branchiopod theme are at least partly due to predator avoidance while continuing to feed on very small suspended and detrital foods.
Taxonomy and Origin of Anostraca – top
When Did Crustaceans and Other Arthropod Subphyla First Appear on Earth?
Fossil ages are generally reported using geologic time units rather than years. Here, the start of the geologic time unit as given by the Geological Society of America’s 2018 “Geologic Time Scale” is considered the age of a fossil assigned to that unit and the geologic unit is given in parentheses. This seems less tiresome than more rigorously reporting the fossil age as somewhere between the older and younger bounds of the time unit and more helpful than just giving the geologic time unit. Consequently, the fossil age is actually “up to” or “no older than” the years given. Fossils from the same geologic time unit could differ in age by millions of years since the major geologic time units (i.e., periods) are up to 80 million years long. They are considered the same age for the purpose of the following discussion. Ages are from Moore (1969) unless otherwise noted. In the case of the Rhynie Chert and its contained fossils, the age given is a precisely determined uranium-lead isotopic age rather than the age of a geologic time unit.
Anostraca Taxonomy Table 1 (based on Moore, 1969, p. R13 and R112-116) illustrates the taxonomic context of Anostraca and uses colors to indicate whether the earliest fossils of a particular group are older than 419 million years (i.e., pre-Devonian, pale blue shading), 419-252 million years old (i.e., Devonian through Permian, pale green shading), 252-66 million years old (i.e., Triassic through Cretaceous, pale yellow shading), or younger than 66 million years old (i.e., Tertiary to present, no shading). Reviewing the fossil ancestry of related taxonomic units is a first step in understanding the origin of Anostraca.
Anostraca Taxonomy Table 1
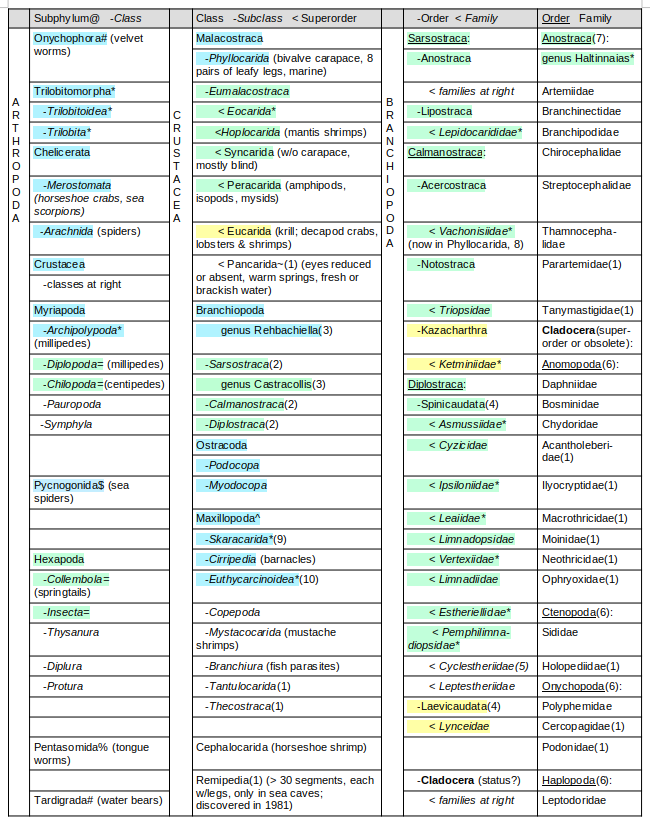
Pale blue – oldest fossils before 419 million years old (before Devonian)
Pale green – oldest fossils 419-252 million years old (before Triassic)
Pale yellow – oldest fossils 252-66 million years old (before Tertiary)
@ Moore (1969) considered these subphyla as superclasses.
* extinct
# Onychophora (e.g., en.wikipedia.org/wiki/Onychophora, onychophora.com) and Tardigrada (e.g., Thorp and Covich, 2001, p. 11) are now considered by some as phyla separate from Arthropoda.
= Chilopoda centipedes, “putative” Diplopoda millipedes, Collembola springtails, and Insecta chewing mandibles have been found in the 412 million years old Rhynie Chert (Dunlop and Garwood, 2017).
$ Pycnogonida is considered a class of Chelicerata by britannica.com or as a separate class of, and not within a subphylum of, Arthropoda by oceanexplorer.noaa.gov.
% Pentasomida is considered a class (or subclass) of Crustacea by some (e.g., en.wikipedia.org/wiki/Pentasomida, encyclopedia.com) or as a separate phylum by others (e.g. de Oliveira Almeida and Christoffersen, 2002).
~ Pancarida is not in Moore (1969) but has only one order, Thermosbaenacea, which Moore (1969) placed in Peracarida.
^ This taxonomic unit (e.g., britannica.com) is not used by Moore (1969) so he considered the following subclasses as classes.
(1) Not in Moore (1969, p. R13 and R112-116).
(2) Belk (1982), following Moore (1969).
(3) Olesen (2007) did not include Rehbachiella within Sarsostraca, Calmanostraca, or Diplostraca and placed Castracollis on the Calmanostraca branch with Notostraca. However, Olesen (2009) kept Castracollis on the same branch as Calmanostraca but did not consider it a member of Calmanostraca.
(4) Identified as orders by Thorp and Covich (2001, p. 850) whereas Moore (1969) has them as suborders of the order Conchostraca.
(5) Identified as order by Olesen (2009) and more closely related to cladocera than to Spinicaudata and Laevicaudata.
(6) Identified as orders by Thorp and Covich (2001, p. 850) but not recognized by Moore (1969), who instead placed all the included families in the order Cladocera. Haplopoda = Leptodora of Olesen (2007).
(7) Rogers (2015a) modified the families listed in Belk (1982) as follows: the genera Linderiella, Polyartemia, and Polyartemiella were moved to Chirocephalidae so Lindariellidae and Polyartemiidae are no longer families, and the genera Parartemia and Tanymastix were moved into their own families Parartemiidae and Tanymastigidae.
(8) As reported by Walossek (1993, p. 69), Vachonisiidae fossils have been reclassified as members of the subclass Phyllocarida.
(9) Fossils of Skaracarida (class Maxillopoda), which was not listed by Moore (1969), are 497 million years old (Upper Cambrian) (as reported by Walossek, 1993).
(10) 444 million years old (Silurian) fossils of Euthycarcinoidea (class Maxillopoda) have been found (McNamara and Trewin, 1993) since Moore (1969).
No arthropod fossils are definitely older than 541 million years although Moore (1969) listed an older “?Precam.” age for Onychophora fossils. 3 other subphyla are up to 541 million years old (Cambrian): Trilobitomorpha, Chelicerata, and Crustacea. That puts Crustacea among the 4 oldest arthropod subphyla. Fossils of all these animals occur in marine sedimentary rocks.
Among crustacean fossils, Moore (1969) listed both ostracods and malacostracans as Early Cambrian but the former has not been recognized by more recent authors. As summarized by Olempska and others (2011), the earliest ostracod of the subclass Podocopa is 470 million years old (Middle Ordovician). Siveter and others (2010) noted that the earliest fossil of the subclass Myodocopa is 458 million years old (Late Ordovician). That leaves the 541 million years old (Early Cambrian) fossils of the subclass Phyllocarida as the oldest crustaceans.
Some arthropod classes are not as old as Crustacea, Trilobitomorpha, Chelicerata, and Onychophora but are still older than 419 million years (blue highlight in Table 1). The oldest myriapod fossil is also the oldest terrestrial fossil and is a 433 million years old (Middle Silurian) millipede of the class Archipolypoda (Wilson and Anderson, 2004). The fossils have spiracles that were used for respiration and occur in sedimentary rocks interpreted as river deposits. Although some have questioned whether Archipolypoda belongs within the class Diplopoda, Wilson and Anderson (2004) considered it separate.
Subphylum Pycnogonida was listed as 419 million years old (Devonian) by Moore (1969) but Siveter and others (2010) reported that 433 million years old (Middle Silurian) fossils have been found.
Additional arthropod classes appeared more recently than 419 million years and before 252 million years ago (green highlight in Table 1). Examinations of the well preserved fossils in the 412 million years old Rhynie Chert have pushed back the ages of some classes. These include centipedes of the class Chilopoda (subphylum Myriapoda), springtails of the class Collembola (subphylum Hexapoda), and insects (subphylum Hexapoda) (Dunlop and Garwood, 2017). Moore (1969) had listed the oldest fossils of these classes as 145 million years old (Cretaceous), Recent, and 323 million years old (Pennsylvanian), respectively. The Rhynie Chert also contains a “putative millipede (Diplopoda)” fossil (Dunlop and Garwood, 2017) while the oldest fossil known to Moore (1969) was 323 million years old (Pennsylvanian).
The remaining arthropod subphyla Pentasomida and Tardigrada, the classes Pauropoda and Symphyla in the subphylum Myriapoda, and Thysanura, Diplura, and Protura in the subphylum Hexapoda haven’t left fossils older than 66 million years old (Tertiary) (Moore, 1969, p. R13).
Taxonomy and Origin of Anostraca – top
When Did Branchiopods and Other Crustacean Classes First Appear on Earth?
The fossil record of Branchiopoda is biased and incomplete because not all branchiopod species have shells or other body parts that are easily fossilized. The carapaces of notostracans and conchostracans are amenable to fossilization but those of cladocerans are not and anostracans have no carapace at all. Nonetheless, soft bodies of branchiopods have been preserved in rare cases due to uncommon geological conditions. The genus Rehbachiella has a carapace but soft body parts have also been preserved. It is the oldest known branchiopod. Fossils of Rehbachiella were discovered in 497 million years old (Late Cambrian) marine sedimentary rocks (Walossek, 1993) since Moore’s (1969) treatise. Olesen (2007) considered Rehbachiella ancestral to other branchiopods rather than belonging to one of the subclasses Sarsostraca, Calmanostraca, or Diplostraca.
Branchiopoda is not the oldest class in the subphylum Crustacea but it is older than most. The oldest crustacean fossils are of the class Malacostraca, sub-class Phyllocarida and are 541 million years old (Early Cambrian). Like branchiopods, they are filter feeders but the appendages used for filter feeding are structured differently and do not provide propulsion (Walossek, 1993, p. 100-105). There are fossils of Skaracarida (crustaceans of the class Maxillopoda) in the same rocks as Rehbachiella.
As mentioned above, ostracods appeared before 419 million years ago but aren’t as old as Moore (1969) had thought. Subclass Podocopa is 470 million years old (Middle Ordovician) and subclass Myodocopa is 458 million years old (Late Ordovician).
Other crustacean subclasses and superorders are younger. Those older than, or possibly older than, 252 million years are
- subclass Cirripedia (class Maxillopoda) from 444 million years ago (Early Silurian),
- subclass Euthycarcinoidea (class Maxillopoda) also from 444 million years ago (Silurian) but less precise (McNamara and Trewin, 1993),
- superorder Eocarida (subclass Eumalacostraca) from 393 million years ago (Middle Devonian),
- superorder Hoplocarida (class Malacostraca) from 359 million years ago (Mississippian),
- superorder Syncarida (subclass Eumalacostraca) from 331 million years ago (Upper Mississippian),
- superoder Peracarida (subclass Eumalacostraca) from 299 million years ago (Permian), and
- superorder Eucarida (subclass Eumalacostraca) possibly from 299 million years ago (Permian) or from 252 million years ago (Triassic).
The remaining crustacean classes and subclasses are much younger. Subclass Copepoda of class Maxillopoda has left 23 million years old (Miocene) fossils. Moore (1969) labeled the class Cephalocarida and the maxillopod subclasses Mystacocarida and Branchiura as “recent”, which probably indicates a lack of fossils or fossils younger than 2.6 million years old (Pleistocene). The class Remipedia and the subclasses Tantulocarida and Thecostraca of the class Maxillopoda hadn’t been identified from extant species by the time of Moore (1969).
Taxonomy and Origin of Anostraca – top
When Did Anostracans and Other Branchiopod Orders First Appear on Earth?
The work of Harvey, Velez, and Butterfield (2011) suggests a pre-488 million years old age and marine origin of sarsostracan-like branchiopods. They obtained carbonaceous microfossils by dissolving 510-488 million years old (age range given by Harvey, Velez, and Butterfield, 2011, for the Middle and Late Cambrian) sedimentary rock in acid. Some have similarities to mandibles of fossil Rehbachiella, fossil Lepidocaris, and extant anostracans and were interpreted as branchiopod-like mandibles. In addition, they found arrays of bristles like those on the leaf-like legs of branchiopods. The host rock is a near-shore marine sedimentary rock.
497 million years old (Late Cambrian) Rehbachiella fossils are the oldest branchiopods known. The clam shrimp families Cyzicidae and Assumiidae of the order Spinicaudata and sub-class Diplostraca, all left 393-419 million years old (Early Devonian) fossils according to Moore (1969). The clam shrimp family Ipsiloniidae, also a spinicaudatan, may be as old but its age is less precise because it is assigned to the Devonian rather than to the Early Devonian. 2 branchiopod genera have been found in the 412 million years old (Early Devonian) Rhynie Chert. The genus Lepidocaris is similar to anostracans but has been assigned to the order Lipostraca, within the sub-class Sarsostraca. The genus Castracollis is similar to notostracans but Olesen (2007, p. 166 and 180) considered it a different order withing the sub-class Calmanostraca. The oldest anostracan and notostracan fossils and another spinicaudatan were coincidentally found at the same locality in 365 million year old (Famennian Age of Later Devonian) rock at the Strud locality in Belgium (Gueriau and others, 2016). These ages indicate branchiopods other than cladocerans got a relatively early start. The sub-classes Sarsostraca, Calmanostraca, and Diplostraca had differentiated by 412 million years ago and the orders Anostraca (fairy shrimp), Notostraca (tadpole shrimp), and Spinicaudata (clam shrimp) by 365 million years ago.
The earliest branchiopods lived in marine habitats. Rehbachiella fossils are the remains of animals that lived close to the sediment-water interface at the bottom of the ocean and have been delicately replaced by phosphate minerals precipitated from phosphorus-rich seawater (Walossek, 1993, p. 3). By 412 million years ago, Lepidocaris and Castracollis were on land. They lived in ponds in a river valley that had hot springs spewing silica-rich water that crystallized to form the Rhynie Chert and trapped animal and plant fossils in the process. The clayey pond sediments at the Strud locality are associated with flood plain deposits, underlain by river-deposited sand, and overlain by mud-cracked sandy silt (Gueriau and others, 2016). Living in the ponds were the anostracan Haltinnaias serrata, the spinicaudatan Gesvesia pernegrei, and the notostracan Strudops goldenbergi. Some contemporary ephemeral pond habitats are similarly populated by anostracans, spinicaudatans, and notostracans (e.g., many ponds in Middle Washoe County, Kibby Flat Playa Lake in Monte Cristo Valley, and Northeastern “Lewiston Lake” in the Antelope Hills).
Cladoceran families did not leave fossils older than 34 million years (Oligocene) according to Moore (1969). Contrary to Moore (1969), Thorp and Covich (2001, p. 871) mentioned cladoceran-like fossils which are 247 million years old (Middle Triassic) and 237 million years old (Late Triassic).
The phylogenetic diagram of Olesen (2007, p. 166 and 180) maps evolutionary origins based on morphologies. It indicates the genus Rehbachiella probably belongs in its own order. Sub-class Sarsostraca is next and is ancestral to the sub-classes Calmanostraca and Diplostraca, which Olesen (2009) labeled as the group Phyllopoda. Sarsotraca includes the orders Lipostraca (fossils only) and Anostraca. Calmanostraca has the extant order Notostraca (tadpole shrimp), the fossil order Kazacharthra, and maybe the fossil genus Castracollis (but Olesen, 2009, had Castracollis branching from Calmanostraca rather than within it). The next branches, which are off the Diplostraca stem, are the clam shrimp orders Laevicaudata and Spinicaudata. That leaves a Cladoceramorpha stem. After the clam shrimp order Cyclestherida branched off the Cladoceramorpha, the 4 extant cladoceran orders follow in succession. Olesen (2009) added an extinct cladoceran order, Cryptopoda, branching off before the extant orders but it is not included in Table 1 because I have no information on the age of the fossils. Olesen’s (2007, 2009) comprehensive view of the branchiopod lineage indicates Anostraca is the oldest of the extant orders although the oldest recovered fossils are probably of the order Spinicaudata.
Taxonomy and Origin of Anostraca – top
How Did Early Anostracans Live?
The fossil record shows more than who is older than whom. In favorable cases, it also reveals biological communities associated with a particular habitat. Extensive studies of the Rhynie Chert in Scotland have documented 412 million years old branchiopods living in ponds with plants and other animals. The University of Aberdeen has made much of this work accessible to the public (start at www.abdn.ac.uk/geosciences/departments/geology/the-learning-resource-1883.php).
Unlike the usual process of gradual compaction of sediment, expulsion of pore water, and mineralization of bone or shell, the Rhynie Chert preserved soft animal and plant parts nearly instantaneously in a very fine grained mass of translucent silicon dioxide, or chert. The Rhynie Chert accumulated near the faulted margin of a river valley where ponds collected in local depressions. Hot springs along the fault occasionally expelled silica-rich water which flowed across the landscape and into some of the ponds. The water cooled rapidly and precipitated chert (Parry and others, 2011; Strullu-Derrien and others, 2016; Dunlop and Garwood, 2017). Analogies have been drawn to the chert terraces around some geysers at Yellowstone National Park and to hot springs in Iceland. Animal or plant parts suspended in the water were essentially frozen in place and are visible as shadings of organic matter within the hard, translucent chert. The chert can be cut into very thin, transparent slices of rock that can be viewed with an optical microscope or a conformal laser-scanning microscope (e.g., Strullu-Derrien and others, 2016). The andesitic volcanism which gave rise to the hot spring system has been dated at 412 million years old using the radioactive decay constants of uranium and the isotopic ratios of uranium and the daughter product lead in zircon minerals from a lava flow or sill (Parry and others, 2011).
If you have a hard time understanding how soft animal parts can be preserved in chert associated with a hot spring, read about the coot fossil less than 10,000 years old that was preserved in a now extinct hot spring terrace pool in Yellowstone National Park (Channing and others, 2004). Because the fossil includes some feathers, encrustation by opal, which is hydrous silicon dioxide, began within 3 days of death (Channing and others, 2004). The fossil shows no signs of trauma. Because coots are unlikely to land in or walk into scalding water, the bird may have been killed by asphyxiation due to methane, carbon dioxide, or hydrogen sulfide (Channing and others, 2004) or by a sudden eruption of the hot spring that caused very hot silica-rich water to flow or rain into a cooler pool where the bird may have been floating. Channing and Edwards (2004) further documented how experimental silicification of plants in an active Yellowstone National Park hot spring “replicated the exceptional three-dimensional preservation characteristic of many Rhynie chert plant fossils”.
Dunlop and Garwood (2017) summarized the terrestrial animals that have so far been found in the chert. There are nematode worms that were probably plant parasites. There are predatory centipedes (class Chilopoda of subphylum Myriapoda) and possible millipedes (class Diplopoda of Myriapoda). The class Arachnida (subphylum Chelicerata) is represented by 5 species of mites of the order Acariformes, a trigonotarbidan with book lungs, and a harvestman (order Opiliones) with tracheal tubes. The book lungs and tracheal tubes are clear evidence of breathing air. For the subphylum Hexapoda, there is a small springtail species (class Collembola) and a probable insect.
The terrestrial plants are not like the conifers and flowering plants we have today but 5 of the genera are considered true vascular plants. The Devonian was still early days for terrestrial plants and the Rhynie plants were generally less than 20 cm (8″) high (www.abdn.ac.uk/geosciences/departments/geology/fossil-flora-1901.php).
In the pond habitats, there are cyanobacteria, unicellular and filamentous algae, charophytes (green algae that differ from chlorophytes and may be the ancestors of land plants), nematophytes (mat-like forms with tubular components of various shapes that may be extinct fungi, algae, or lichens), and the earliest known lichen (www.abdn.ac.uk/geosciences/departments/geology/fossil-flora-1901.php). There are several types of fungi, and Strullu-Derrien and others (2016) have imaged and described fungal rhizoids and zoosporangia associated with 2 different substrates.
The Rhynie ponds are also home to a species of the crustacean subclass Euthycarcinoidea. Although found in rock with pond fauna and flora, they have 10 pairs of jointed legs that are not at all suitable for swimming. They are believed to have fed on organic detritus (www.abdn.ac.uk/geosciences/departments/geology/euthycarcinoids-1953.php).
Strullu-Darrien found spheroids about 50 micrometers in diameter (0.05 mm, 0.002″) which have surfaces that are covered by spines up to 8 micrometers (.008 mm, 0.0003″) long. They interpreted these as resting eggs of a branchiopod species. Resting eggs are key to the survival and dispersal of branchiopods. If the spiny spheroids are branchiopod eggs, they indicate branchiopods had accomplished that critical adaptation before 400 million years ago.
The branchiopods found in the Rhynie Chert are Lepidocaris rhyniensis, classified as a lipostracan, and Castracollis wilsonae, which is a calmanostracan or is closely related to calmanostracans. They are small: 3 mm (0.1″) for Lepidocaris and 8 mm (0.3″) for Castracollis. Lepidocaris is clearly a sarsostracan because it lacks a carapace and has 11 pairs of legs. It differs in some respects from extant anostracans (www.abdn.ac.uk/geosciences/departments/geology/lepidocaris-rhyniensis-1962.php) but why it couldn’t be an early anostracan is not explained. Importantly, the “general ecology of Lepidocaris may have been somewhat similar to that of modern anostracans” (www.abdn.ac.uk/geosciences/departments/geology/lepidocaris-rhyniensis-1962.php).
The shield-like carapace of Castracollis distinguishes it as a likely calmanostracan. It has 21-26 segments with paired legs and up to 28 leg-less segments. The last 10-15 pairs of legs are smaller and decrease in size toward the anus (www.abdn.ac.uk/geosciences/departments/geology/castracollis-wilsonae-1963.php). The lesson from the Rhynie Chert is that sarsostracans and calmanostracans had already adapted to ponds with foods like algae and cyanobacteria, and co-existed with fungi and a variety of terrestrial and aquatic plant and animal species by 412 million years ago.
433 million years old (Middle Silurian) non-marine beds have fish (Wilson and Anderson, 2004) so the branchiopods living at the time of deposition of the Rhynie Chert were probably already excluded from streams and large lakes. By 393 million years ago (Middle Devonian), fish had developed the ability to populate lakes subject to strong seasonal fluctuations and longer term wet-dry cycles and hence could have preyed on branchiopods even in moderately unstable lake environments. This was elucidated by Scottish geologists who studied lake sediments of the Orcadian Basin in the Orkney Islands (Leather, 2021). Repeating cycles of different sedimentary rock types within the Rousay Flagstone Formation indicate strongly variable seasonal precipitation with persistent lakes at times and ephemeral playa lakes or no lakes at other times. Several fossil fish species occur in strata associated with persistent lakes. It is also noteworthy that spinicaudatan fossils of the family Ipsiloniidae were found with fish near the base of the Rousay Flagstone Formation (Leather, 2021). The absence of fish fossils in the Rhynie Chert, consequently, doesn’t mean that fish had not evolved by that time, it means that the branchiopods of the Rhynie Chert had already found their way into fishless habitat.
Fossils in 365 million year old sediments at the Strud locality confirm branchiopods’ early adoption of ephemeral pond habitats (Gueriau and others, 2016). As mentioned above, the branchiopod fossils occur in clayey pond sediments rather than in sandy river sediments. Further evidence of branchiopod avoidance of fish and flowing streams is the presence of placoderms (armored fish; en.wikipedia.org/wiki/Placodermi), actinopterygians (ray-finned fish like many species living today; en.wikipedia.org/wiki/Actinopterygii), and acanthodians (jawed fish like sharks; en.wikipedia.org/wiki/Acanthodii) in river sediments below but not in the pond sediments. Figure 1 and Figure 4 of Gueriau and others (2016) suggest the branchiopods mostly have the ponds to themselves although “decapods, eurypterids, a putative insect, and plant macrofossils” were found in adjacent flood plain shales.
Importantly, what appear to be spinicaudatan and notostracan eggs also occur in the pond sediments. Their identification as branchiopod eggs is more convincing than for those in the Rhynie Chert because some occur under the carapaces of the spinicaudatans and notostracans, as in contemporary species. This suggests branchiopods as a group had developed resting eggs adapted to ephemeral habitats before 365 million years ago. Gueriau and others (2016, p. 387): “It is more parsimonious to suppose an ancestral acquisition of resting eggs in Branchiopoda in tandem with colonization of ephemeral pools [44], and long before the morphological specialization of each subclade, rather than convergent evolution of these attributes.” Eggs associated with spinicaudatans had a mean diameter of about 0.16 mm but those within carapaces of notostracans were only about 0.07 mm in diameter. Gueriau and others (2016, p. 388) speculated that the smaller size of the notostracan eggs was an adaptation to wind dispersal whereas 100-145 million year old notostracan egg fossils and contemporary eggs that are more than 4 times bigger indicate stronger adaptation to dispersal by ingestion of adult branchiopods with eggs and then defecation of the eggs into a different pond.
If branchiopods evolved in the marine environment first, as suggested by Rehbachiella and the Middle to Late Cambrian branchiopod-like mandibles, how did they get from the sea or a coastal lagoon to a pond? And how many times did that have to happen before a branchiopod population was able to survive the dramatic salinity drop and produce eggs that could hatch in brackish or fresh water?
Taxonomy and Origin of Anostraca – top
The Great Game: Extinction and Survival
A great many arthropod and crustacean families developed in the period 541 to 299 million years ago (Cambrian-Pennsylvanian) but most went extinct. At the higher subphylum level, only the subphylum Trilobitomorpha has gone completely extinct (see Anostraca Taxonomy Table 1). At the class level, Archipolypoda bit the dust, perhaps literally as they are millipedes. Of crustaceans, animals of the superorder Eocarida and of the subclass Euthycarcinoidea are no longer with us.
Several branchiopod families have gone extinct. They include:
the sarsostracan family
- Lepidocarididae (known from 419-393 million years ago, Early Devonian);
the calmanostracan families
- genus Castracollis (family not formalized), which is a calmanostracan or is closely related to them (known from 419-393 million years ago, Early Devonian);
- genus Strudops (family not formalized) (known from 372-359 million years ago, Late Devonian); and
- Ketmeniidae (known from 201-174 million years ago, Early Jurassic); and
the conchostracan families
- Assumiidae (known from 419-66 million years ago, Early Devonian-Late Cretaceous),
- Ipsiloniidae (known from 419-101 million years ago, Devonian-Early Cretaceous).
- Leaiidae (known from 393-101 million years ago, Middle Devonian-Early Cretaceous),
- Vertexiidae (known from 359-201 million years ago, Early Carboniferous-Late Triassic),
- Pemphilimnadiopsidae (known from 323-299 million years ago, Pennsylvanian), and
- Estheriellidae (known from 323-101 million years ago, Late Carboniferous-Early Cretaceous).
Such is life.
Knowing when various species appeared in the fossil record and when they died off may be the less interesting part of the story. Who survived? The few long-lived, surviving crustacean families include:
- Trypetesidae (class Cirripedia) from 359 million years ago (Carboniferous),
- Penaeidae (subclass Eumalacostraca of Malacostraca) from 299 million years ago (Permian) or from 252 million years ago (Triassic),
- Nebaliidae (subclass Phyllocarida of Malacostraca) from 273 million years ago (Late Permian),
- Anaspididae (subclass Eumalacostraca of Malacostraca) from 252 million years ago (Triassic),
- Sphaeromatidae (subclass Eumalacostraca of Malacostraca) from 252 million years ago (Triassic), and
- Amphisopidae (subclass Eumalacostraca of Malacostraca) from 252 million years ago (Triassic).
Three branchiopod families have survived for more than 299 million years from the start of the Permian and all 3 appeared before 5 of the 6 other long-lived crustacean families above. They are:
- the conchostracan Cyzicidae from 419 million years ago (Early Devonian),
- the conchostracan Limnadopsidae from 359 million years ago (Early Carboniferous), and
- the notostracan Triopsidae from 323 million years ago (Pennsylvanian) (Moore, 1969, p. R112-R116).
Remarkably, 252 million years old (Triassic) fossils in Germany have been identified as the extant notostracan species Triops cancriformis (Moore, 1969, p. R134).
In contrast, the oldest cladoceran fossils are only 34 million years old (Oligocene) according to Moore (1969, p. R113) but possibly 240 million years old (Middle Triassic) according to Thorp and Covich (2001, p. 871). The cladoceran Daphniidae family appeared 34 million years ago (Oligocene) (Moore, 1969, p. R113) and is common to this day.
The fossil record thus indicates that branchiopods are some of the hardiest crustaceans. By adapting to challenging environments with characteristics that restrict or exclude colonization by many other species, they have developed robust survival strategies. At least some conchostracan and notostracan families survived the great mass extinctions at 252 million years ago (end Permian) and 66 million years ago (end Cretaceous) and one extant species of notostracan survived the 66 million years ago event, which is famous for killing off the dinosaurs. Hardy resting eggs surely played a role in the survival of these families. Anostracans produce hardy resting eggs, too. Maybe some anostracan families are similarly long lived but have not left fossils.
So there. You have something to ponder the next time you find fairy shrimp, tadpole shrimp, or clam shrimp swimming in some pond some where. They have mastered the art of life in a changing world with evolving predator species. How about you?