Fairy Shrimp Birth
Fairy Shrimp Growth
Fairy Shrimp Reproduction
___ Fairy Shrimp Reproductive Life Span
___ Fairy Shrimp Sex Ratio
___ Fairy Shrimp Mating Dance
___ Fairy Shrimp Mate Recognition
___ Fairy Shrimp Sexual Selection
___ Fairy Shrimp Egg Production
Non-reproductive Behaviors of Fairy Shrimp
Fairy Shrimp Death
Fairy Shrimp Birth
What hatches from a fairy shrimp egg isn’t a fairy shrimp; it is a nauplius. Nauplius is another name for larva (Johansen, 1921, p. 22) and could be the accepted term for crustacean larvae. “It is often only the size of a pin-head” (Johansen, 1921, p. 22). The first nauplius stage of the genus Artemia is 0.35 mm (0.014″) long (Walossek, 1993, p. 86). Like the young of other arthropods, fairy shrimp nauplii have bodies unlike adults. The huge increase in body size to adulthood and changing shape is accomplished by molting. The nauplius grows, molts by discarding its exterior covering, and becomes the next instar with more and bigger appendages, which eventually become legs. Antennae II are used for swimming in the early stages. Without legs, the earliest stage(s) may not feed at all but survive on their yolk. Different species have 14-18 instars, which I presume are the results of successive molts. Within a single species, the number of instars may change with environmental conditions (Pennak, 1978, p. 335-336). When the nauplius begins to have adult characteristics it is called a metanauplius by some (Johansen, 1921, p. 22). Nauplii are succeeded by juveniles, which look like adults but aren’t sexually mature.
Fairy shrimp eggs are discussed more fully on the Resting Eggs of Fairy Shrimp and Their Hatching page.
Life Cycle of Fairy Shrimp – top
Fairy Shrimp Growth
Like other animals, fairy shrimp spend much of their lives growing. In some cases, growth rates are slow for the first few days or even weeks. This could be due to the limited feeding abilities of early nauplii or to a paucity of phytoplankton for fairy shrimp hatching in the winter or spring. Once fairy shrimp are more than about 2 mm long, they grow relatively rapidly until they reach sexual maturity. Then growth rates slow or stop.
A sampling of growth rates, how long it takes to reach sexual maturity, and maximum lengths (biased by publication availability) is given in the table “Pre-Maturity Growth Rates and Mature Lengths of Fairy Shrimp”. The most common pre-maturity growth rates are 0.4-1.1 mm/day for the populations shown.
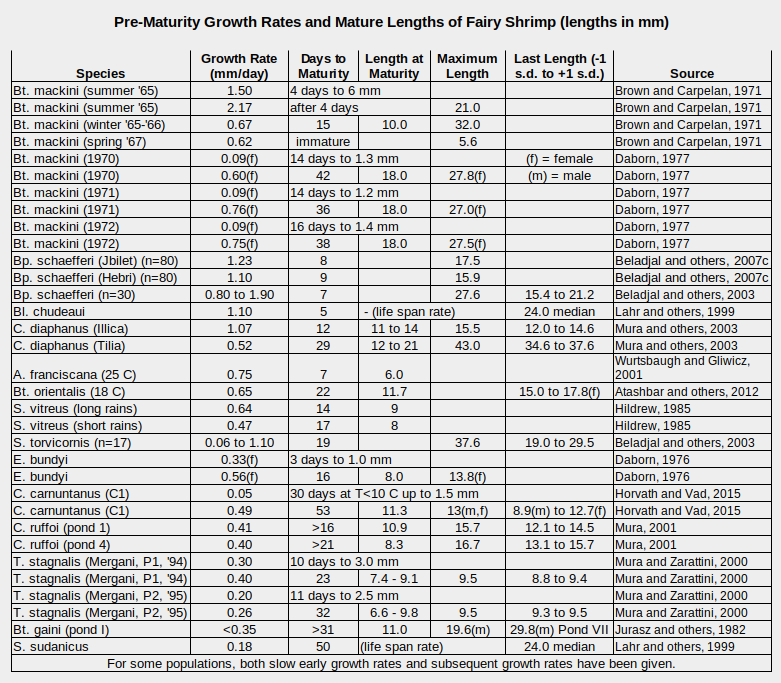
f-female, m-male
A. – genus Artemia, Bl. – genus Branchinella, Bp. – genus Branchipus, Bt. – genus Branchinecta, C. – genus Chirocephalus, E. – genus Eubranchipus, S. – genus Streptocephalus, T. – genus Tanymastix
Growth rates can differ by a factor of 2 or more for the same species in the same or different ponds, such as the rates reported by Brown and Carpelan (1971) for B. mackini, by Beladjal and others (2003) for B. schaefferi, and by Mura and others (2003) for C. diaphanus. An early winter generation of Eubranchipus holmani in a pond in Louisiana had growth rates of 0.87 mm/day for the first 9 days and a later generation had growth rates of only 0.20 mm/day for the first 14 days (Moore, 1963).
Examples of very slow early growth rates were given by Horvath and Vad (2015) for C. carnuntanus and by Daborn (1977) for B. mackini in Canada. Temperature may have been a factor as Daborn (1976) found that a pause in the growth of Eubranchipus bundyi in Alberta 16-19 days after hatching coincided with a drop in temperature.
Fairy shrimp that live long enough experience a late slow down in growth. Brown and Carpelan (1971, p. 43) inferred that the B. mackini they observed reached maturity at lengths of about 10 mm, at which point growth stopped while the gonads developed. Growth subsequently resumed at a slower rate but was not measured. In Moore’s (1963) Louisiana pond, the growth rate of the early winter generation of E. holmani slowed from 0.87 mm/day to 0.32 mm/day after 9 days.
As shown in the table “Pre-Maturity Growth Rates and Mature Lengths of Fairy Shrimp”, a few studies reported that the longest observed fairy shrimp was female but for Branchinecta gaini in Antarctica, it was a male. Some found that the mean lengths of males and females were significantly different but the studies I have read are too few to draw general conclusions.
Fairy shrimp reach sexual maturity in a few days to a few weeks. In addition to the data in the table, Maynard and Romney (1975) reported that Branchinecta packardi in Utah matured in 15 days. In some studies, Artemia franciscana took 18 days to reach sexual maturity (Pennak, 1978, p. 336). Eubranchipus holmani took 22-46 days in Louisiana, based on weekly counts by Moore (1969). Rogers (2015b) reported the number of days from pond inundation to first observation of ovisacs with fertilized eggs for several species in California. Branchinecta sandiegonensis reached maturity the fastest. It took only 3 days in November 2021. The slowest was the large predator Branchinecta gigas, which took 31 days in March 2006. Others species took 27, 21, 19, 17, 16, 12, and 6 days (Rogers, 2015b). In laboratory experiments designed to mimic natural conditions, Artemia monica in “Mono Lake” reached sexual maturity in 57 or 62 days under cool spring-like temperatures, with high or low food supply respectively, and 24 days under summer-like temperatures and low food supply (reported in Jones and Stokes Associates, 1993b, p. J-2).
Of course, the growth rates discussed above apply to the fairy shrimp that lived long enough to be measured. In a few cases where someone addressed the question of juvenile deaths, it was found that most fairy shrimp die young in the natural environment. Quantitative sampling of Chirocephalus carnuntanus in Hungary revealed that the density of juveniles on March 25 was only 20% of the density of nauplii on March 1 and the density of adults on April 10 was only 7.7% of the density of nauplii on March 1 (Horvath and Vad, 2015). Predators were not identified but could have been present. In the observations of Conover and Caudell (2009), less than 3% of Artemia franciscana nauplii in “Great Salt Lake” became adults. “Great Salt Lake” has no fish or amphibians and few insect predators. Would birds bother with fairy shrimp less than 5 mm long or did they die of something other than predation?
In laboratory settings, juvenile survival rates seem to be much higher. The mortality of pre-reproductive Branchipus schaefferi was 1.24% per day and that of Streptocephalus torvicornis was 0.33% per day (Beladjal and others, 2003). 50% of both species survived until day 19 or day 82, respectively (Beladjal and others, 2003). Survival rates were greater than 60% on day 20 for Artemia urmiana and A. parthenogenetica from Iran in the better tolerated TDS concentrations (Agh and others, 2008) and greater than 70% for Artemia franciscana from 5 Mexican lakes on day 21 in the better tolerated TDS concentrations (Castro-Mejia and others, 2011).
Life Cycle of Fairy Shrimp – top
Fairy Shrimp Reproduction
Fairy Shrimp Reproductive Life Span
Fairy Shrimp Sex Ratio
Fairy Shrimp Mating Dance
Fairy Shrimp Mate Recognition
Fairy Shrimp Sexual Selection
Fairy Shrimp Egg Production
Fairy Shrimp Reproductive Life Span
Those fairy shrimp which survive to sexual maturity can start reproducing. It would be very difficult to determine how sexually active males are but, in the laboratory, it is easy to count how many clutches of eggs individual females produce. Most, but not all, females do.
Hildrew (1985) found that in his experiments, some Streptocephalus vitreus females “failed to produce repeat clutches, even when kept with males” but that seems to imply they did produce at least one. In the experiments of Yang and Sun (2023), 48-80 Artemia sinica females were paired with males and observed individually for each experimental treatment and essentially none reproduced at the 2 highest TDS concentrations at temperatures of 16 C and 30 C. However, that may have been due to unfavorable experimental conditions rather than due to a lack of sexual interest. In the best case (25 C, 100,000 mg/L TDS, 18 hours light per day), 4% of females did not reproduce (Yang and Sun, 2023). For Artemia franciscana females hatched from eggs that were 24, 16, or 2 years old, the percentages of non-reproducing females were 30%, 40%, and 6%, respectively, in the experiments of Rode and others (2011).
At least some, and probably most, females in natural environments continue reproducing until death. There are several egg-filled ovisacs visible among these remains of fairy shrimp on the dried mud of Garfield 5890 Saddle Pond. Daborn (1977) observed that once about 40% of Branchinecta mackini females carried eggs, that percentage did not drop below 40% as long as the population lasted.
In experiments allowed to continue until the fairy shrimp died “naturally”, Branchipus schaefferi females continued reproducing until death (Beladjal and others, 2003). Streptocephalus torvicornis females were also said to have no “postreproductive” period but the last one died at 125 days and the reproductive period ended on “day 100”, or possibly day 119 allowing for 19 days to reach sexual maturity (Beladjal and others, 2003). In TDS-temperature experiments, mean “post-reproductive” periods of up to 2 weeks were reported by Agh and others (2008) for Artemia parthenogenetica and “post-reproductive” ranges of 0-35 days by Yang and Sun (2023) for Artemia sinica. Figure 3 of Atashbar and others (2012) shows Branchinecta orientalis females had mean “post.rep” periods of a few days at all experimental temperatures but no numbers were given.
Back to Fairy Shrimp Reproduction
Fairy Shrimp Sex Ratio
Anostracans typically have female to male ratios of about 1:1 (Rogers, 2019). However, this is a misleading indication of the availability of females because to a female fairy shrimp, mating only makes sense if it has unfertilized eggs ready to be released into the ovisac. Once eggs have been fertilized, it may take a few to several days before the next batch is ready. Consequently, the number of available, or receptive, females in a population is inversely proportional to the length of time it takes to produce the next clutch. One way to improve the chances of successful egg fertilization under these circumstances would be to have more females than males. Mura (2001) suggested that female-biased ratios may be advantageous in less predictable, short-duration ponds whereas male-biased ratios would be more likely in predictable, long-duration ponds and cited various references to support that view. The implication seems to be that maximizing egg production has some evolutionary advantage in short-duration ponds but maximizing competition among males is better in long-duration ponds.
A greater percentage of females than males has been observed in some fairy shrimp populations. This is shown in the table “Percentages of Females in Various Fairy Shrimp Populations”. This table is not representative of all fairy shrimp species. It just has the ones I could download information on. The ratio often changes over the lifespan of the population due to changing death rates for males and females. The unusual case of Streptocephalus torvicornis in the table is a result of all the females dying within 125 days of birth but some males living for 325 days in the experiment of Beladjal and others (2003). Additionally, Johansen (1921, p. 23) observed “far more females than males” in ponds in the Arctic and sub-Arctic of Canada and Alaska.
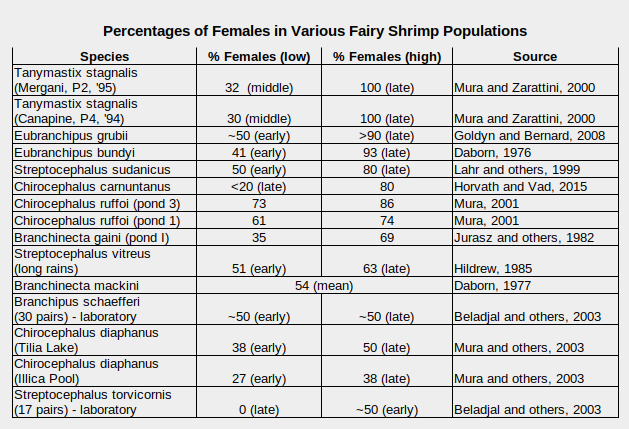
Populations of Artemia franciscana which are native to the Americas but invasive in southern France have screwed up their sex ratios. They are “aberrant” (Lievens and others, 2016, abstract only). These populations have “extremely male-biased sex ratios”. Experiments have demonstrated that A. franciscana females produce male eggs in proportion to the number of female fairy shrimp in the pond. The invasive populations coexist with parthenogenetic Artemia populations, which are entirely female. As a result, the invasive A. franciscana populations sex ratios reflect the total number of Artemia females in the pond regardless of species (Lievens and others, 2016, abstract only). How the invasive reproductive females determine the overall proportion of females in the pond is not mentioned in the abstract but pheromones seem likely. In spite of its aberrant sex ratios when co-existing with parthenogenetic Artemia, A. franciscana has become the dominant Artemia species in coastal lagoons and salt works of Portugal, Cadiz Bay in Spain, and the Mediterranean coast of France and it outcompetes parthenogenetic Artemia in laboratory experiments (Amat and others, 2005).
Parthenogenetic populations of fairy shrimp have only females. They produce eggs that don’t need to be fertilized. Much of what follows doesn’t apply to them.
Back to Fairy Shrimp Reproduction
Fairy Shrimp Mating Dance
The fairy shrimp mating dance has the following movements.
- Searching by both females and males (“detection and orientation” of Wiman, 1981; omitted by Tapia and others, 2015; #2 male mate searching and #3 hill topping of Rogers, 2019).
- Following by males and acceptance or swimming away by females (“station taking” of Wiman, 1981; approach of #1 “approach and touch” of Tapia and others, 2015; #4 male orientation and #6 female swim away of Rogers, 2019).
- Clasping by males and acceptance or evasive jerk by females (same as Wiman, 1981; touch of #1 “approach and touch” and #3 “riding attempt” of Tapia and others, 2015; #5 male touch and #6 female swim away of Rogers, 2019).
- Copulation (“intromission” of Wiman, 1981; #3 “riding success” of Tapia and others, 2015; #7 “amplexus” of Rogers, 2019).
- Disengagement (same as Wiman, 1981; omitted by Tapia and others, 2015; #8 male disengagement of Rogers, 2019).
- Opening of gonopore, or not, by females (omitted by Wiman, 1981; omitted by Tapia and others, 2015; #9 “female may open her gonopore” of Rogers, 2019).
To understand fairy shrimp mating behavior, it is important to distinguish receptive females from non-receptive females. Belk (1984) defined a receptive female as one “having an empty ovisac and mature ova in lateral oviduct pouches”. Similarly, Rogers (2019) defined receptive females as those which have eggs in the “lateral pouches” rather than in the “brood pouch”. I have serendipitously photographed lines of white eggs on either side of the ovisac at Bivouac Lake and “Coyote Lake”. In both photographs, there are also brown eggs in the ovisacs that have been shelled. Consequently, the photographed females were non-receptive.
Fairy shrimp are always swimming so specific mating behavior is hard to identify in the field. By observing Branchinecta lindahli individuals in the laboratory and classifying their behaviors into 5 categories and 3 water depths, Rogers (2019) showed that hormonally conditioned males raised in the presence of females and non-conditioned males raised in the absence of females differed in the amounts of time they spent engaging in various behaviors. The most striking differences were that female-aware males spent 22% of their time in detection/avoidance and 1.5% of their time in not swimming while female-naïve males spent 0% of their time in detection/avoidance and 23% of their time in not swimming. Receptive and non-receptive females showed similar differences in detection/avoidance and not swimming behaviors. In addition, receptive females strongly preferred surface water to bottom water (66% of time vs. 1.5% of time) while non-receptive females strongly preferred bottom water to surface water (92% of time vs. 0% of time). This is significant for mating because in the following movement, males swim under the females. That becomes awkward if the female is less than 2 cm from the bottom of the container.
Swimming more actively and engaging in detection and avoidance of other fairy shrimp can plausibly be related to searching for mates (#1). Not swimming isn’t. Scraping surfaces is unlikely to be related to mating and time spent doing that behavior did not differ substantially between sexually aware and sexually naïve males and females.
Following behavior (#2) can be recognized in the field. Following behavior continues for at least a few seconds but probably not more than 15 seconds (EIGWUU). Males approach other fairy shrimp from below and behind to set themselves up for clasping. If the following male discerns that the followed individual is not a receptive female, it will go look for another fairy shrimp. A followed non-receptive female would swim away from the follower. How followers are detected has not been investigated but it is probably visual. The 2 fairy shrimp in “North Gap Lake” East Pond appear to be engaged in following. In this case, they both appear to be males (i.e., no ovisacs) and will soon go their separate ways. What is perhaps most notable about the “Alkali Lake” videos (Choreography of the Clear Water Crowds in “Alkali Lake” on the Fairy Shrimp Videos page) is the lack of following. A few of the female fairy shrimp look like they have a few white eggs but they could all be non-receptive.
The clasping movement (#3) results in direct contact between following males and followed females. The male swims close to the female and positions its head below the female’s genital segments at the end of its thorax (Belk, 1984, describing Eubranchipus serratus). The male extends its long antennae II around the body of the female and grasps the female’s genital segments (Belk, 1984). If the male has antennal appendages like E. serratus, it places them along the female’s back (N.B., both swim with backs toward the bottom of the pond) (Belk, 1984). If the female accepts the proposal, it may curl its body briefly but then relaxes and swims slowly (Belk, 1984). If the female does not want to mate, it executes sharp jerking motions by flexing its thorax and abdomen in order to dislodge the male. This is usually successful. In Belk’s (1984) experiments with E. serratus, 79% of mating attempts were unsuccessful and all failures were attributed to female rejection. It usually took less than 6 seconds to dislodge the male but took more than 16 seconds in a few cases. Belk (1984) did not report any instances of jerking motions failing to eventually shake off an unwanted male.
In the experiments of Tapia and others (2015) with Artemia franciscana, “riding success” was about 25% as common as “riding attempt” (their Figure 2). The 2 categories are mutually exclusive (i.e., all “riding attempts” were failures). Consequently, the success rate may have been about 20%. All “riding attempt” failures were due to female behavior. Tsai and others (2017) observed 32 “amplexus” attempts, which I interpret as clasping, by Branchinella kugenumaensis in an experimental setting and 6 “intromission” events, for a success rate of 19%. Artemia males are more tenacious claspers. Belk and Serpa (1992) observed several Branchinecta campestris females attempt to dislodge Artemia franciscana males over a period of about 30 minutes but only one succeeded.
Life Cycle of Fairy Shrimp – top
It may be a general rule that males of the genus Artemia extend the clasping movement into a mate-guarding chorus. Kaestner (1970, p. 88) considered the purpose of Artemia pairs remaining clasped together for hours as facilitating multiple copulations, which seems rather pointless. In contrast, Forbes and others (1992, citing Lochhead, 1950) interpreted prolonged pairing for 1 to several days as a way for male Artemia to time copulation with the female’s molting cycle, which is synchronized with its reproductive cycle (e.g., Weeks and Benvenuto, 2008), and to prevent access by other males. Zapata and others (1990) also noted couples that remained paired for “up to several days”. Rode and others (2011) indicated that some Artemia franciscana pairs remained coupled for more than 8 days. I have so commonly seen coupled Artemia pairs that I have come to consider it diagnostic of the genus.
Artemia males are not the only fairy shrimp which practice mate-guarding. Most males of Artemiopsis stefanssoni that Johansen (1921, p. 29) saw were clasping females, even in October under 7 inches (18 cm) of ice. He deduced that males clasping females for long periods of time prior to copulation “is perhaps a necessary procedure as there are generally far more females than males” (Johansen, 1921, p. 23). Elsewhere in the Arctic, Polyartemiella hazeni and Branchinecta paludosa made representative sampling difficult because “the individuals or attached pairs often hovered in rows” (Stross and others, 1980, p. 254).
Mate guarding has been documented in another branchiopod, the clam shrimp Eulimnadia texana (Benvenuto and Weeks, 2012). In this species, the females are hermaphrodites and can make their own sperm but do mate with the less abundant males from time to time. Males and females may remain attached for up to 2 hours. In the experiment, the longest mean guarding duration, 60 minutes, occurred when the females were “relaxed” by treatment with magnesium sulfate and consequently less able to shake off male suitors. The mean duration was 12 minutes for the control without treatment (Benvenuto and Weeks, 2012).
If the male isn’t shaken off and is not mate-guarding, copulation (#4) follows immediately after clasping. The male’s left and right penes are located on the genital segments at the rearward end of its thorax. The male curls the end of its thorax upward and forward to reach the female’s gonopore at the forward end of the ovisac, which is above the male’s head. The male’s body generally forms a U- or J-shape (Belk, 1984). E. serratus males additionally bend their abdomens, which are rearward of the penes, back away from the female to create an S posture (Belk, 1984). Sperm is transmitted through the penes to the gonopore. Copulation takes about 2 minutes for E. serratus (Belk, 1984). “Intromission” by Branchinella kugenumaensis takes 1.6-3.4 seconds (Tsai and others, 2017). Artemia franciscana engages in “riding success” for 5 minutes (Tapia and others, 2015).
Disengagement (#5) naturally follows copulation. Belk (1984) observed that Eubranchipus serratus females disengaged with “one or more rapid jerking movements”. Rogers (2019) suggested that males may disengage during clasping “depending on the fit of the amplexus”. That would presumably abort the mating process. The female and male return to swimming normally after disengagement.
Rogers (2019) identified a final step (#6) in mating wherein the “the female may open her gonopore”. If the female doesn’t, the sperm would presumably swim off into the pond water rather than into the ovisac. This suggests that females have one last chance at rejection if swimming away after following or evasive action during clasping didn’t work.
If the female opens its gonopore, the fertilization process begins and the female becomes non-receptive. Eggs are pushed from the oviducts into the ovisac. There, shell gland secretions cover the eggs with shells. At some point, the ovaries begin producing more eggs.
The mating dance of fairy shrimp doesn’t always follow the standard choreography. I saw 2 A. franciscana males hanging on to the same female in “Steamboat Lake” 2nd East Pond (Granite Mountains). Forbes and others (1992) also occasionally found 2 males attached to the same female when the population density was high. I’ve also seen an Artemia male attached to the female backwards so that it and the female were pointing in opposite directions. The 2 sort of drifted slowly in the direction the female was going.
Figure 2 of Tapia and others (2015) shows a small but non-zero frequency of male-male “riding success” (i.e., copulation). I can’t picture what that means.
In spite of the evidence that Streptocephalus torvicornis, Branchipus schaefferi, Branchinecta paludosa, and Polyartemiella hazeni can distinguish the pheromones of different species of fairy shrimp (see below), some fairy shrimp mate with a different species anyway. As noted above, A. franciscana males were found clasping B. campestris females. Belk and Serpa (1992) did note that the pond where the pairs were collected had a very high density of fairy shrimp. When males of Branchinecta belki were placed in the same containers as females of Branchinecta packardi and when males of Branchinecta packardi were placed in the same containers as females of Branchinecta belki, viable eggs were produced in both cases (Maeda-Martínez and others, 1992). The same authors cited other successful hybridization experiments with the genus Streptocephalus and between Artemia monica and Artemia franciscana. Natural hybrids of Branchinecta lindahli and B. sandiegoensis have been found in southern California (Simovich and others, 2013). According to Simovich and others (2013), “Rogers (2002b) found that males of all species of Branchinecta tested were willing to amplex with females of other species of Branchinecta”. Maeda-Martínez and others (1992) cited Wiman’s (1979b) proposal that an efficient mate recognition process (“premating isolating mechanism”) may be lacking in some closely related species. Could it be that the long evolutionary history of Anostraca has determined that, contrary to what many biologists think, hybridization is not such a bad thing? Alternatively, natural hybridization could be too rare to matter.
Back to Fairy Shrimp Reproduction
Fairy Shrimp Mate Recognition
Although mate recognition is probably obvious to fairy shrimp, it is not so obvious to humans. How do they do it?
“Anostracan males use imprecise visual cues to guide their mating behavior” (Belk, 1991, abstract only). The experiments of Tsai and others (2017) lend some support to that claim if not full corroboration. They compared the frequencies of mating behaviors under full lighting with a 14 watt fluorescent lamp and dark lighting with blue cellophane covering the fluorescent lamp. “Orientation” (i.e., following movement, #2), “station-taking” (i.e., positioning for clasping, #3), and “amplexus” (i.e., clasping movement, #4) behaviors occurred much less frequently (p less than 0.001 for each) under the dark conditions. Tsai and others’ (2017) experimental design did not distinguish between the effects of mood and the ability to see other fairy shrimp. And what of the few who did mate in the dark? How did they do it?
Rogers’s (2002, cited by Rogers, 2019) observation that males performed the clasping movement (“amplexing”) with dead females suspended on string also supports the importance of vision.
Fairy shrimp that live in opaque water necessarily rely less on vision for mating but I haven’t read any descriptions of mating in opaque water. That’s one of the few things Brown and Carpelan (1971) omitted from their treatise on Branchinecta mackini.
“Anostracan females use tactile cues provided largely by outgrowths of the male’s second antennae in choosing mates” (Belk, 1991, abstract only). Whether or not Belk did additional experiments on tactile cues in 1991, his experiments in 1984 support this claim. Belk (1984) compared the copulation rates of Eubranchipus serratus males which had had their antennal appendages amputated with intact males and found them to be 5% (n=250) and 21% (n=253), respectively. Success was determined by whether the female shook the clasper off or not so the sensation of the antennal appendages on the females’ backs is what made the difference. The antennal appendages did not effect the ability of the females to dislodge the males. It took less than 6 seconds to dislodge 65% of the amputated males and 64% of the intact males (Belk, 1984).
The smell of pheromones in the water can be added to visual and tactile cues as a possible means of mate recognition. Beladjal and others (2007b) proved the presence of pheromones that could be detected by eggs but it was not in the context of mating. They showed that resting eggs did not hatch when placed in filtered water that had contained their parents in the laboratory. That a chemical in the water was the culprit was confirmed when Streptocephalus torvicornis eggs finally hatched after the parents’ water was diluted to 25%. That the egg hatching response was specific to species was indicated by moderate numbers of S. torvicornis and B. schaefferi eggs hatching in water that had had Chirocephalus diaphanus, i.e., 30% and 80% of the control hatching rate, respectively (Beladjal and others, 2007b). The presence of Chirocephalus diaphanus likely has no ecological significance to S. torvicornis and B. schaefferi eggs as it does not occur in the same ponds at the same time as these species (Beladjal and others, 2007b). Inhibition of hatching by the parents’ pheromones could have developed as a means of population control or as a signal that the succeeding pond duration would be shorter than otherwise.
Other species distinguish between species-specific pheromones. Polyartemiella hazeni and Branchinecta paludosa in an arctic pond “were segregated one from another in the mating swarms” (Stross and others, 1980, p. 254). Pheromones are a likely explanation for this phenomenon but there are other possible causes.
2 approaches have shown that pheromones are a strong control of mating by fairy shrimp. Males soaked in a polar compound extracted from water in which virgin females of Artemia franciscana had been living elicited nearly the same mating behaviors in normal males as the presence of virgin females did (Tapia and others, 2015). In the tests, 1 soaked male or virgin female was placed in beakers with 1 or 5 normal males and observed for 1 hour. Most convincingly, mean frequencies of “riding attempts” (i.e., clasping not followed by copulation) were equal (with 1 normal male) or slightly lower (with 5 normal males) for soaked males than for virgin females and were much lower for males which had not been soaked with female water (Figure 2, Tapia and others, 2015).
Life Cycle of Fairy Shrimp – top
In the second approach, female-naïve males of Branchinecta lindahli were raised in the absence of female B. lindahli and male-naïve females of B. lindahli were raised in the absence of male B. lindahli to exclude the potential influence of pheromones. The times individual fairy shrimp subsequently spent engaging in various behaviors was recorded. In addition, water which had contained females was added to containers with female-naïve males and their subsequent behaviors were observed. The behaviors of the treated fairy shrimp were compared to those of normal males which had been raised with females and normal receptive females which had eggs ready to be fertilized. Of the behaviors recorded by Rogers (2019), I consider “detection/avoidance” (including pursuit) of other fairy shrimp as a positive indicator of mate searching behavior, “not swimming” as a negative indicator of mate searching, and “scraping” container surfaces for food as unrelated to mate searching.
Comparing the percentages of times engaged in the aforementioned behaviors between normal receptive females vs. male-naïve females (Rogers, 2019):
- Detection/avoidance – 21% vs. 0%.
- Not swimming – 2.9% vs. 12%.
- Scraping – 7.4% vs. 4.8%.
This looks like good evidence for male pheromones.
Comparing the percentages of times engaged in various behaviors between normal males vs. female-naïve males:
- Detection/avoidance – 22% vs. 0%.
- Not swimming – 1.5% vs. 12%.
- Scraping – 8.2% vs. 15.8%.
This looks like good evidence for female pheromones.
Comparing the percentages of times engaged in various behaviors between normal males vs. female-naïve males after the addition of female-inhabited water to containers of female-naïve males:
- Detection/avoidance – 22% vs. 22%.
- Not swimming – 1.5% vs. 1.9%.
- Scraping – 8.2% vs. 6.7%.
This looks like even better evidence for female pheromones.
Rogers’s (2019) ad hoc modification of his pheromone experiments further demonstrated the importance of pheromones. Female-naïve males did not increase mate searching behaviors when females in glass bowls were placed floating in their containers. When the glass bowl of females was presented after water that had contained females was added to the container of the female-naïve males, the female-naïve males did approach the females in the glass bowl. Visual cues alone are not sufficient. It takes both visual and olfactory cues for the mating dance.
Back to Fairy Shrimp Reproduction
Fairy Shrimp Sexual Selection
Visual, tactile, and chemical cues provide female and male fairy shrimp with the means to select mates. Do they practice sexual selection?
- Belk (1984) would only go so far as to write that “antennal appendages play an important, but not exclusive, role in mate recognition”.
- Wiman (1981, abstract only) was unequivocal – “[M]ales discriminate against clearly unsuitable mates both before and during station-taking”.
- Belk (1991, abstract only) finally said it: “[e]volution of . . . elaborate male courtship structures [i.e., male antennae II and appendages] seems to be . . . driven by sexual selection by female choice”.
One example of sexual selection is by size. In the laboratory at least, large male Artemia franciscana prefer large females and vice versa. Forbes and others (1992) found good and mediocre correlations between female and male body sizes in 2 samples of coupled pairs: r = 0.74 (n=16, p less than 0.005) and r = 0.42 (n=12, p less than 0.10). They also discovered that when single large and small females and males were placed in beakers in various combinations, the large males and large females coupled sooner than the other 3 combinations. In the experiments of Forbes and others (1992), large males swam around faster and encountered females at higher rates. This increases the probability of large males mating but not necessarily with large females. Thus, female choice is also likely in play. The benefits of pairing with large females was evident from a strong correlation between lengths of females and the numbers of eggs in their clutches (Forbes and others, 1992). The benefits of large male size, if any, were not determined.
As indicated by Belk (1991, abstract only), the strongest case for sexual selection in fairy shrimp is the superficially weird and complex structures of male antennae II and their appendages. Having described the male antennae II of Eubranchipus gelidus (now Eubranchipus bundyi – Rogers, 2013), Johansen (1921, p. 27) wrote of “broad, lobated appendages, which we may suppose are used for ‘tickling’ “. In addition to the larger structures, the basal segments of the antennae II, which are in contact with the female’s genital segments during clasping, have protuberances, spiny areas, stout outgrowths (Linder, 1941, p. 130), bulges with denticles, “pulvillus covered with minute spinules”, “peg-like protuberance”s (Lynch, 1964), rows of spines, large and small pads covered with minute spines, spine-bearing bulges and cylindrical processes, and spiny ridges (Belk, 1975). These would clearly evoke tactile responses by females even if that were not their primary role. With such offerings, why wouldn’t females be choosy? The characteristics of male antennae II are the most common discriminants used in species identification (Belk, 1975). In fact, evolution of antennae II and appendages could be the dominant cause of speciation as selection by females sets up a competitive feedback loop. Belk (1984) was “inclined to hypothesize” as much but advised caution.
The elaborate structures of male antennae II do not appear to offer any adaptive advantage. They are not used in male-male combat like the oversized antlers and horns of some large hoofed animals. They impose greater water resistance that likely makes swimming slower or more tiring. Although held away from the legs, they could interfere with feeding to some extent. Whether they could signal some kind of male fitness is of course unknown.
The relatively small and simple male antennae II of the species Branchinecta paludosa (Figures 229A and 239B, Pennak, 1978, p. 329, 341) demonstrate that elaborate, oversized male antennae II are not necessary for species viability. B. paludosa seems to be successful. It is widely dispersed throughout the Arctic and down into the middle latitudes, like Montana (Lynch, 1958) and Wyoming (Horne, 1967). B. paludosa is also versatile. It inhabits tundra ponds (Lindholm and others, 2016), alpine ponds (Jons Snowy Range Pond), sagebrush steppe ponds (“Coyote Lake” in the Antelope Hills), and rock pools (Lankin Dome Summit Rock Pool #3 in the Granite Mountains). Lynch (1958) found it on the “plains” of Montana at an elevation of 1,280 m (4,200′). Branchinecta cornigera has similarly simple antennae II and occurs in eastern Washington (Lynch, 1958).
Back to Fairy Shrimp Reproduction
Fairy Shrimp Egg Production
Fertilized eggs are the successful outcome of the mating dance. Fairy shrimp produce lots of them. It’s possible some eggs don’t get fertilized due to wayward or incompetent sperm but I haven’t found any mention of that. Females held in the laboratory without access to males “produced smooth, blue, shiny eggs which rapidly decomposed” (Hildrew, 1985).
Eggs are retained in the ovisac for one to several days before being expelled into the water. Daborn (1977) reported 14 days for Branchinecta mackini in Alberta. Or, the eggs remain in the ovisac until the female dies (Pennak, 1978, p. 334). The ovisac of Artemiopsis stefanssoni does not have an opening (Daborn, 2011, abstract only) so eggs are retained until the ovisac falls apart after the female’s death. In species of fairy shrimp with female reproductive cycles of only a few days, fertilized eggs are probably all released over a short span of time. However, the large range in the number of eggs in the ovisacs of older females of Chirocephalus ruffoi led Mura (2001) to conclude that they do not release all their eggs at once.
The variations of egg production between species, between populations of the same species, between individuals in the same populations, and between early and late clutches of the same females are so great that averages cannot tell the tale. In some cases, standard deviations exceed the means, rendering neither statistic appropriate. I report ranges where available.
A sampling of egg counts is presented in the table “Numbers of Eggs per Clutch and per Lifetime in Fairy Shrimp”. The short answer to the question “How many eggs do individual female fairy shrimp produce?” is “Probably between 0 and 5,000”. The large standard deviation ranges of various reproductive measures in the table for fairy shrimp of the same species in water with the same experimental conditions indicate generalizations are largely meaningless. Ranges in the table are 0 to 368 eggs per clutch, 0-25 clutches per lifetime, 1.2 to 30 days between clutches, and 0 to 5,090 eggs per lifetime. Female fairy shrimp in the same environment can experience life very differently.
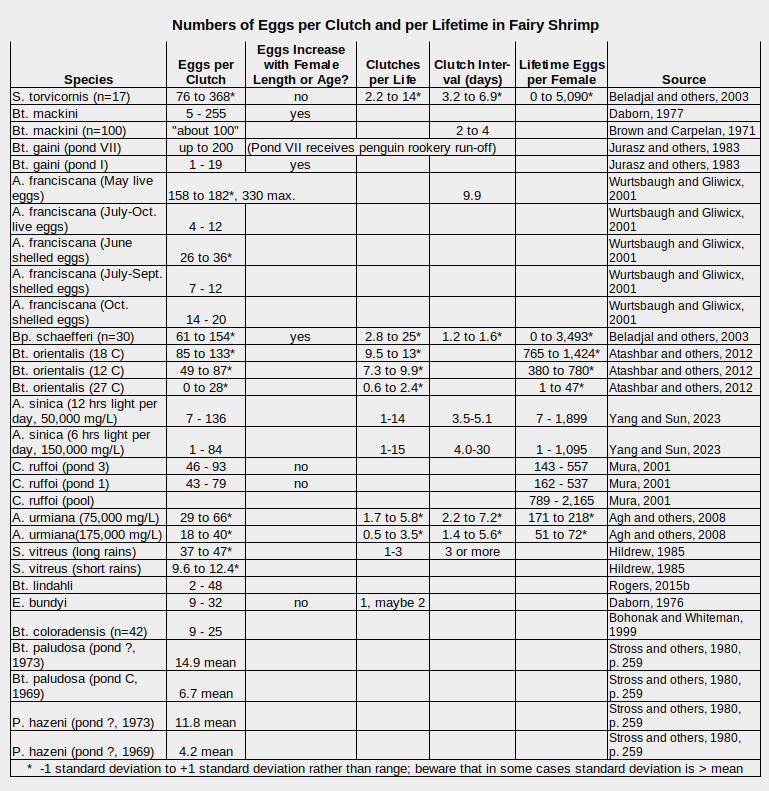
A. – genus Artemia, Bp. – genus Branchipus, Bt. – genus Branchinecta, C. – genus Chirocephalus, E. – genus Eubranchipus, P. – Polyartemiella, S. – genus Streptocephalus
It may or may not help to point out that the data of Stross and others (1980) are for Arctic fairy shrimp and those of Jurasz and others (1982) are for Antarctic fairy shrimp. The Branchinecta coloradensis of Bohonak and Whiteman (1999) lived in the mountains of Colorado at elevations of about 3,400 m (11,150′). The Branchinecta mackini of Daborn (1977) were in Alberta while those of Brown and Carpelan (1971) were in the Mojave Desert of California. Rogers’s (2015b) B. lindahli were from both southern and northern California. The C. ruffoi ponds are in the mountains of southern Italy at elevations of about 1,740 m (5,700′). The experiments with B. schaefferi, S. torvicornis, and A. sinica were at 25 C and those with A. urmiana at 27 C.
Life Cycle of Fairy Shrimp – top
My own 2 anecdotes are:
- More than 4 dozen eggs in Streptocephalus seali at a lower and warmer (19 C) elevation of 2,380 m (7,800′) and less than 2 dozen eggs in Branchinecta coloradensis at a higher and cooler (12 C) elevation of 3,330 m (10,900′), both in the Wind River Mountains, and
- More than 5 dozen eggs at a lower and warmer (20 C) elevation of 1,805 m (5,920′) in the Garfield Hills and less than 20 eggs at a higher and probably cooler elevation of 2,225 m (7,300′) in the Pine Grove Hills.
There are many reasons for differences in egg production. An observation by Jurasz and others (1982) identified one that isn’t often measured. They noted that the fairy shrimp in pond VII were larger and the females carried far more eggs than those in pond I. Although pond VII is smaller than pond I, it is down hill from a penguin rookery and has more fairy shrimp food, principally algae, because of the fertilized run-off.
The data of Wurtsbaugh and Gliwicz (2001) illustrate an adaptation to a permanent lake that gets colder than 5 C in the winter. They are based on successive observations of Artemia franciscana in “Great Salt Lake”. That population produces both live eggs that hatch within a few days and shelled eggs that develop into resting eggs that hatch in the next or succeeding years. Large numbers of live eggs are produced by the first generation in May. The moderate numbers of shelled eggs produced in June are probably also by the first generation. Small clutches of live and shelled eggs continue to be produced through September, likely by both the first and second generations. Somewhat larger clutches of shelled eggs are produced by the lake’s second generation in October.
Like “Great Salt Lake”, “Mono Lake” is a permanent lake with high TDS and cold winter temperatures. There, the clutch sizes of Artemia monica increase with both female body length and the abundance of algal food, as indicated by the concentration of chlorophyll-a (Stokes and Associates, 1993). The overall proportion of egg-bearing females increases with temperature and body length through the summer but the proportion of females with live eggs decreases with temperature and body length (Stokes and Associates, 1993). This is because in the cooler spring, females produce mostly live eggs and have bigger clutches the longer they get and as the algae continue to proliferate. As the larger females of the first generation die off and algal productivity declines over time, the mostly second generation females produce smaller clutches of shelled eggs.
As shown in the temperature section on the habitats page, water temperature has a noticeable effect on egg production. The graph Fairy Shrimp Egg Clutches vs. Temperatures for Eggs Collected in Iran shows how egg production for an experimental population of Branchinecta orientalis was greatest at 18 C and decreased at both lower and higher temperatures. Both clutch sizes and numbers of clutches per lifetime (not shown) were well correlated with the lifetime egg totals. Shorter lives at 24 C and 27 C contributed to the drop off in egg production.
The Habitats of Fairy Shrimp page presented 3 graphs showing the variation of egg production with TDS:
Fairy Shrimp Egg Clutches vs. TDS for Artemia urmiana in Iran,
Fairy Shrimp Egg Clutches vs. TDS for Artemia parthenogenetica in Iran, and
Fairy Shrimp Egg Clutches vs. Hours of Light and TDS for Eggs from “Yuncheng Lake”, China.
For Artemia urmiana and A. parthenogenetica, clutch sizes and total lifetime eggs generally decreased as TDS increased from 75,000 to 175,000 mg/L while the number of clutches per lifetime didn’t change much. The graph for A. sinica in “Yuncheng Lake” is complicated by inconsistent variations due to different periods of experimental lighting but an overall downward trend in clutch sizes and total lifetime eggs from 50,000 mg/L to 200,000 mg/L is still evident.
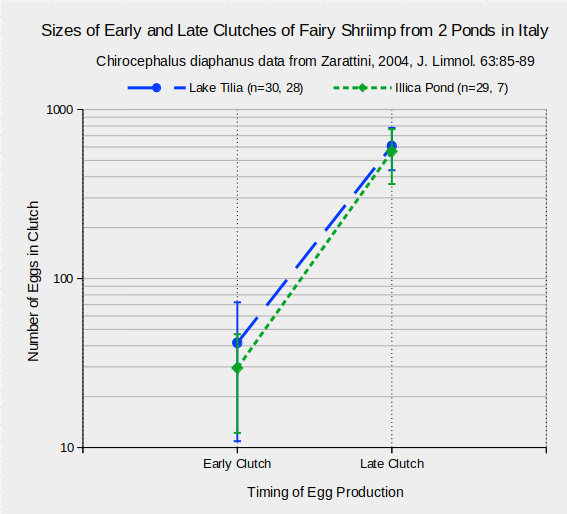
As multiple clutches are common among fairy shrimp in general, do clutch sizes change with age? In 2 cases, dramatic increases in clutch sizes with age have been documented. Sizes of early and late clutches of Chirocephalus diaphanus females collected from 2 ponds in the Central Apennines Mountains of Italy are shown in the graph “Sizes of Early and Late Clutches of Fairy Shrimp from 2 Ponds in Italy”. The increase in clutch size is so extreme, it is shown on a logarithmic scale. Mean sizes of early and late clutches for Lake Tilia fairy shrimp were 42 and 608 and those for Illica Pond were 30 and 564 respectively. Variance of the clutch sizes was also large. The standard deviation for Illica Pond late clutches was 202, which gives a coefficient of variation of 36%.
Life Cycle of Fairy Shrimp – top
Clutch sizes of females of Artemia sinica from “Yuncheng Lake” increased by 1.3 to 5 times from the first to last clutches (Yang and Sun, 2023). Only the ratios of the last clutch sizes to the first clutch sizes, rather than raw egg counts, were given by Yang and Sun (2023). These are plotted in the graph “Comparison of 1st and Last Egg Clutches of Fairy Shrimp in China vs. Hours of Light and TDS” only for the trials with 6 or 18 hours of light and TDS concentrations of 50,000, 100,000, or 150,000 mg/L. The ratios of the 1st clutch sizes to the 1st clutch sizes, which are 1 by definition, are also plotted to make the display comparable to that of Zarattini’s (2004) data. Only the standard deviations for the trials at 50,000 mg/L are shown because they are all so extreme, and effectively meaningless.
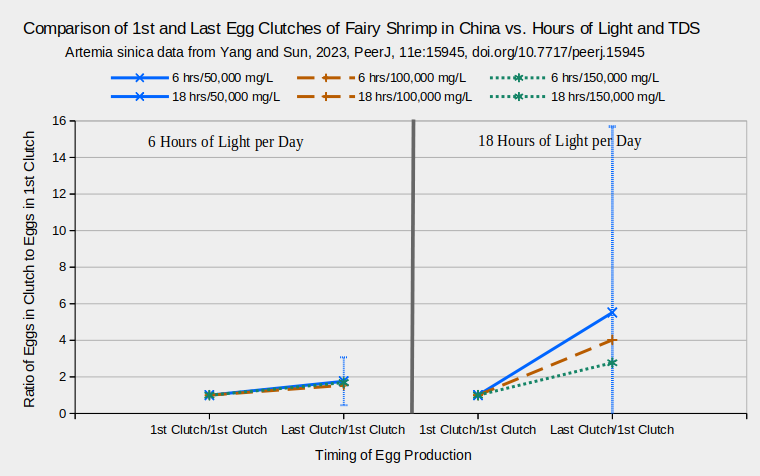
A. monica in “Mono Lake” also produce bigger late clutches but the effect is not so dramatic as the 2 cases above. Linear equations were fit to experimental data for the first clutch (coefficient of determination 0.85) and for the mean of subsequent clutches (coefficient of determination 0.61) (equations in Table J-1 of Jones & Stokes Associates, 1993b). Solving the equations for a TDS concentration of 100,000 mg/L, which is close to the actual TDS of the lake, gives 38 eggs for the first clutch and 62 for later clutches. The equation, unfortunately, gives no hint of how wide the range or standard deviations may be.
Back to Fairy Shrimp Reproduction
Life Cycle of Fairy Shrimp – top
Non-reproductive Behaviors of Fairy Shrimp
Apart from the work on mating, not much has been written (that I can find and access) about other fairy shrimp behaviors. I saw fairy shrimp swimming in clusters (pinkish gray masses) in Small Rare Plant Habitat Pond on April 24, 2019 (Pine Grove Hills). This may be similar to what Hildrew (1985) described for fairy shrimp in Kenya rain pools: “Streptocephalus vitreus appeared to swim in shoals”. I saw densely concentrated Artemia in “Steamboat Lake” 2nd East Pond on June 7, 1989 and in “Piaya Lake” on June 8, 1989 (both in the Granite Mountains) but don’t know if they were less dense in other areas.
“Mono Lake” has dense concentrations of Artemia monica at “sublacustrine springs” and in “plumes” (Winkler, 1977). Compared to normal summer densities of about 1,300 fairy shrimp per cubic meter (35 cubic feet), sublacustrine springs had 38,000 fairy shrimp per cubic meter and plumes had about 200,000 fairy shrimp per cubic meter (i.e., 0.2 individuals per cubic centimeter or about 3 per cubic inch). Winkler (1977, p. 59) thought greater densities of algae or of organic matter in methane seeps might have attracted fairy shrimp to the sublacustrine springs. Their abundance in plumes could be due to “the warming of littoral waters by solar radiation in combination with a photo- and rheotaxic-response by the brine shrimp” (Winkler, 1977, p. 59).
Rogers (2019) listed several examples of various fairy shrimp “congregations” he had seen. He interpreted them all as “hill-topping” mating behavior by analogy with insect swarms. Fairy shrimp swarms in ponds without submerged features that could be analogous to hills were also attributed to mating behavior. In experiments concerning pheromones in Branchinecta lindahli, Rogers (2019) put rocks in aquariums to simulate “hills” and did observe sexually active fairy shrimp spending more time swimming around the rocks than elsewhere.
Swarming behavior may be common in arctic ponds investigated by Stross and others (1980). “During mating . . . the fairyshrimp [sic] of each species [Polyartemiella hazeni and Branchinecta paludosa] moved to one place in the pond, formed pairs, and hovered in large rafts immediately beneath the surface” (Stross and others, 1980, p. 256). In this case, though, the swarming apparently occurred after pairing. Nonetheless, the swarms could consist of fairy shrimp both searching for mates and those paired with mates.
In addition to clustering behavior, fairy shrimp also exhibit non-clustering or spreading out behavior. This is evident in “Alkali Lake” Fairy Shrimp Video 2023-07-06c-r (Alkali Valley) on the Fairy Shrimp Videos page (Choreography of the Clear Water Crowds). The wide spacing and varied swimming directions of individual fairy shrimp seem too consistent to be unintentional. This could be a defensive behavior that evolved to confuse predators and make it difficult for them to choose which individual to eat. It would also make it more difficult for large predators like birds to catch more than 1 fairy shrimp at a time. Gulls visit and avocets nest at “Alkali Lake”.
Unusual swimming patterns of individual fairy shrimp may be specific behaviors. As an example of what is not unusual, the single female fairy shrimp in South Sister Dead Cow Pond Fairy Shrimp Video 2022-06-22b-r (Sweetwater Mountains) on the Fairy Shrimp Videos page (Swimming the Sweetwater Mountains) swims around in an unremarkable search pattern. In contrast, the fairy shrimp in Macari East Stop Sign Pond Fairy Shrimp Video 2022-01-27r (Fairy Shrimp Videos, Clay Dancers of “Carson Lake” Playa) engages in swimming motions that seem to go beyond what could be considered normal feeding or exploring behavior. The same is true of the fairy shrimp in South Sister Hidden Camp Pond Fairy Shrimp Video 2022-06-22a-f (Fairy Shrimp Videos, Swimming the Sweetwater Mountains).
Scraping behavior is described on the feeding page.
Life Cycle of Fairy Shrimp – top
Fairy Shrimp Death
Fairy shrimp don’t live forever or even for very long. Importantly, how fairy shrimp die says a lot about how they live.
Some examples of fairy shrimp life spans are given in the table “Life Spans of Adult Fairy Shrimp in Natural and Laboratory Settings”. One might expect species which mature the quickest to die the soonest but the table doesn’t offer much support for that hypothesis. Like other life characteristics of fairy shrimp, maturation and life spans vary widely between and within species. This is partly the result of their evaporating pond habitats but there are many other physical, chemical, and biological factors that affect life spans.
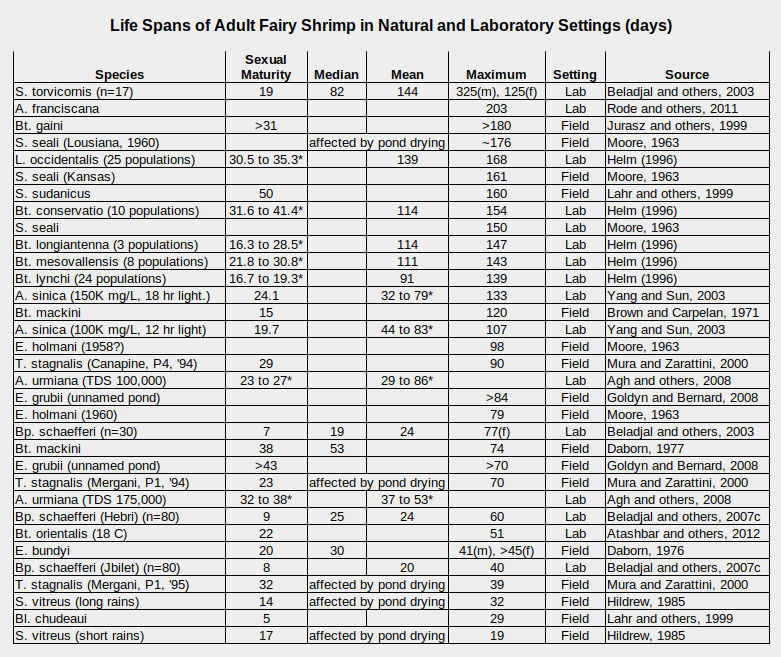
*-1 standard deviation to +1 standard deviation rather than range
A. – genus Artemia, Bl. – genus Branchinella, Bp. – genus Branchipus, Bt. – genus Branchinecta, E. – genus Eubranchipus, S. – genus Streptocephalus, T. – genus Tanymastix
On the other hand, the “live fast, die young” meme is somewhat supported by observations of 5 species of California fairy shrimp. The fairy shrimp were raised in plastic pools of 140 cm (55″) diameter that mimicked a natural setting. The pools were “seeded” with soil from ponds known to have fairy shrimp and allowed to develop in response to natural precipitation and temperatures. Any fairy shrimp that hatched were not fed. All pools had abundant tadpoles and many had predators, such as dytiscids, notonectids, and odonatans. The 2 species with shortest and longest mean life spans matured in less than 20 or more than 30 days (Helm, 1996) as shown in the graph “Mean Days to Maturity and Mean Life Spans of 5 Species of California Fairy Shrimp Raised in Plastic Pools”. However, the species that is slowest to mature has an intermediate life span. The correlation between maturation period and maximum longevity was significant at the 95% confidence level nonetheless.
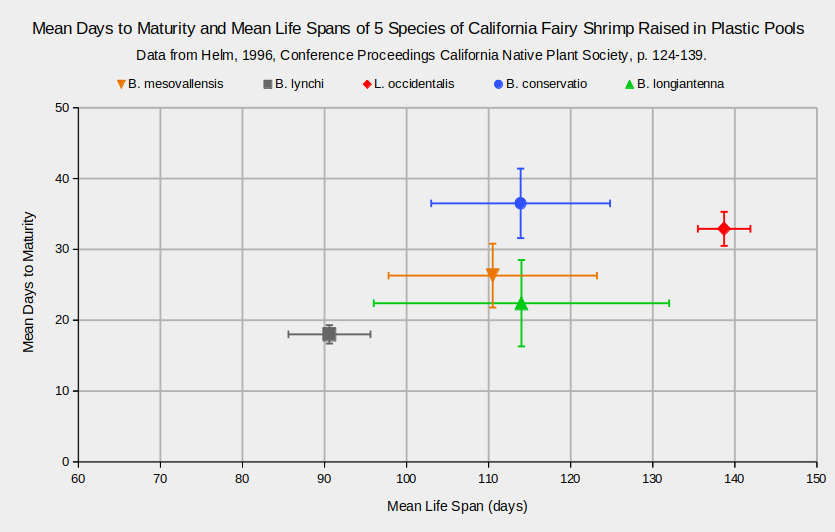
B. – Branchinecta, L. – Linderiella
Error bars represent standard deviations.
Days to maturity were determined from populations raised in plastic pools: 8 pools for B. mesovallensis, 24 for B. lynchi, 25 for L. occidentalis, 10 for B. conservatio, and 3 for B. longiantenna.
Plastic pools 140 cm in diameter were “seeded” with soil from ponds known to have fairy shrimp and allowed to develop in response to natural precipitation and temperatures. Any fairy shrimp that hatched were not fed. All pools had abundant tadpoles and many had predators, such as dytiscids, notonectids, and odonatans.
There are conflicting reports of sexual differences in life spans. In “Mono Lake”, Artemia monica males outnumber females by a 2:1 ratio (as reported by Jones and Stokes Associates, 1993b). In Alberta ponds however, males died off first in a population of Eubranchipus bundyi (Daborn, 1976) and “slightly earlier than females” in a population of Branchinecta mackini (Daborn, 1977). As shown in the table, B. schaefferi females outlived males but S. torvicornis males lived far longer than the females in the experiments of Beladjal and others (2003).
Life Cycle of Fairy Shrimp – top
In evaporating ponds, some fairy shrimp hang on until the last few millimeters of water. Examples occurred in Smith Creek Ranch Road Long Ditch Ponds in March 2013, Burnt Cabin Summit Playa Lake in June 2019, Edwards Creek Playa Lake in May 2023, and Beauty Peak East Pond in April 2021. Others die at the edges of ponds that still have ample water like those at Stinking Springs Well Pond in February 2022 and those in the videos of Digging in the Shallows of Soda Spring Valley on the Fairy Shrimp Videos) page.
In some cases, the increase in TDS as water evaporates may kill fairy shrimp while there are still several centimeters of water remaining in the pond. This appears to be the case at Monitor Playa Lake in July 2019. The larger branchinectid fairy shrimp were swimming listlessly (e.g., female at lower left) or dead while a population of smaller Artemia fairy shrimp (e.g., pink individual at upper right), which are more tolerant of high TDS, were swimming actively.
Increasing water temperatures can also shorten the lives of fairy shrimp, as discussed in the temperature section of Habitats of Fairy Shrimp. In the laboratory, the life span of Chirocephalus grubei was inversely correlated with temperature. It lived about 90 days at 5.2 C, 78 days at 7.9 C, and 50 days at 13.5 C (Kaestner, 1970, p. 89). The life spans of Branchinecta orientalis from Iran similarly decreased with temperature above 15 C in the laboratory.
Although death by desiccation is a common risk in many fairy shrimp habitats, fairy shrimp face a different threat in the far North – being frozen in ice. Johansen (1921, p. 24) “observed how a great number of phyllopods [fairy shrimp] in the fall freeze into the ice as the latter begins to form and grows in thickness”. Nonetheless, some survive in water below ice. In the Northwest Territories of Canada on October 6, 1915, Artemiopsis stefanssoni was swimming in about 13 cm of water under 18 cm of ice (Johansen, 1921, p. 29).
Johansen’s (1921) observations were tested experimentally. Adult Branchinecta gaini were collected from ponds near the British Antarctic Survey’s research station in Antarctica and gradually cooled to -3 C in 1-liter beakers of pond water (Hawes and others, 2008). “Freezing of the surrounding water is a ‘snap’ event with animals swimming freely one moment, encased, and immobile, the next” (Hawes and others, 2008). When gradually reheated to 5 C after 6 hours at -3 C, 93% of the fairy shrimp began to move again after the ice melted. However, 80% of males and 93% of females were dead within the following 48 hours. How did more than 5% of males and females actually survive? And for how long?
Evaporation and freezing are 2 of the things water can do to terminate the lives of fairy shrimp and wind spray is a third. Fairy shrimp also die by “being thrown up along the margin of the particular lake by waves in windy weather” (Johansen, 1921, p. 24). I saw this at Mud Springs Pond #1 in the Great Divide Basin of Wyoming. The dark reddish gray scum on the water is a mass of Artemia individuals blown to the edge of the pond and there are little windrows of dried out Artemia on the shoreline.
One might suppose that fairy shrimp would suffocate or become exhausted in opaque, clay-rich water but those living on “Carson Lake” Playa and in Candelaria Playa Ponds seem to like it (Cruising the Clay-Water Boundary Layer on the Fairy Shrimp Videos page). The clay soup of West Northumberland Road Pond #7 on August 30, 2023 (Big Smoky Valley) may have crossed a threshold though as I didn’t see fairy shrimp there.
Fairy shrimp can take steps to reduce some life-threatening risks. They can survive by swimming at the surface if the water has low oxygen levels (Belk and Cole, 1975) or at depth if the near-surface water is too hot. Fairy shrimp often hatch in large numbers in what may be a cicada-like you-can’t-eat-us-all strategy. Fairy shrimp are often somewhat camouflaged with colors similar to those of the pond bottoms but that is unlikely to help a majority of a population. Birds and other predators find them anyway. The presence of birds at opaque ponds suggests that they can also find fairy shrimp in opaque waters.
For fairy shrimp that die before their pond gets too salty or too hot or dries up, there are a variety of possible causes. Being eaten or dismembered by a predator is certainly probable but the great abundance of fairy shrimp in some ponds and the rarity of predators in many ponds suggest those deaths may not be the most common type.
Life Cycle of Fairy Shrimp – top
I haven’t read any accounts of starvation.
There is the possibility of disease but I haven’t read of any. Fairy shrimp are afflicted by parasites (e.g., Sanchez and others, 2016) but parasitism in the cited case was beneficial, not deadly. Short life spans lessen the chances for cancer. The lack of arteries eliminates the risk of heart attack. No lungs, no pneumonia.
Too much algae in the pond can be a problem. Shantz (1905) noted that Branchinecta coloradensis in “Dead Lake” on July 29 had so many cells of green algae stuck to the bristles on their legs that they swam “with slow labored movements”. Most fairy shrimp died within the next 2 weeks. Shantz (1905) caught only one on August 12 “after a diligent search”. The lake was a few degrees warmer on August 12 as well. Something similar may explain what I found at Smith Creek Highway 50 Well Pond in early May 2023. A small puddle isolated from the main pond was thick with green algae and thicker with dead fairy shrimp. Algal or cyanobacterial toxins or low oxygen concentrations are other possible causes of death for those fairy shrimp.
As in other animals, aging may be a problem. Moore (1969) observed that Eubranchipus holmani “displays increasing evidence of senility with an accompanying high mortality rate following the initial phase of maximum egg production”. Such evidence included slow swimming and damaged appendages. Maybe senility can explain the deaths in the videos of Digging in the Shallows of Soda Spring Valley (Fairy Shrimp Videos). Other individuals seemed to be doing fine.
Fairy shrimp do a pretty good job of not crashing into rocks where I have seen them in rocky ponds like Bivouac Lake. However, in “Squaw Flat Playa Lake Fairy Shrimp Video 2023-04-16-cr” (see “Little Fish Lake” Valley Isn’t Just for Fish on the Fairy Shrimp Videos page) and in Smith Creek Cold Springs Ponds Fairy Shrimp Video 2021-04-23r (see Crowded Cohabitants of Smith Creek Cold Springs Ponds on the Fairy Shrimp Videos page), fairy shrimp occasionally collided with the walls of the plastic white container I had put them in. This is an unnatural hazard though as fairy shrimp are not likely to encounter featureless white walls in real ponds. Fortunately, the fairy shrimp did not appear to be injured by their collisions with the container.
Life itself is straightforward for fairy shrimp. They swim. That’s good for respiration, feeding, moving toward more food, moving away from slow predators, moving toward thermally attractive locations, and just exploring the pond. Swimming comes naturally, right out of the egg. If they get hungry, they just keep swimming. If it gets hot, they just keep swimming. If the water gets a bit salty, well, they just keep swimming. There’s not much they can do about it. Fairy shrimp maintain their self-reliance and self-sufficiency simply by swimming. They don’t have much brains so they probably don’t realize they should be bored. They aren’t likely to worry about the future or remember stupid things they did in the past. If you need guidance for living in the present, look at fairy shrimp.
Life Cycle of Fairy Shrimp – top